Mitochondrial tRNA modifications: functions, diseases caused by their loss, and treatment strategies
- PMID: 39719325
- PMCID: PMC11874988
- DOI: 10.1261/rna.080257.124
Mitochondrial tRNA modifications: functions, diseases caused by their loss, and treatment strategies
Abstract
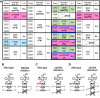
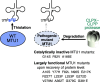
Mitochondrial tRNA (mt-tRNA) modifications play pivotal roles in decoding and sustaining tRNA stability, thereby enabling the synthesis of essential respiratory complex proteins in mitochondria. Consequently, loss of human mt-tRNA modifications caused by mutations in the mitochondrial or nuclear genome can cause life-threatening mitochondrial diseases such as encephalopathy and cardiomyopathy. In this article, we first provide a comprehensive overview of the functions of mt-tRNA modifications, the responsible modification enzymes, and the diseases caused by the loss of mt-tRNA modifications. We then discuss progress and potential strategies to treat these diseases, including taurine supplementation for MELAS patients, targeted deletion of mtDNA variants, and overexpression of modification-related proteins. Finally, we discuss factors that need to be overcome to cure "mitochondrial tRNA modopathies."
Keywords: MELAS; mitoTALEN; mitochondrial disease; tRNA modification; tRNA modopathy.
© 2025 Chujo and Tomizawa; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures

Similar articles
-
Human transfer RNA modopathies: diseases caused by aberrations in transfer RNA modifications.FEBS J. 2021 Dec;288(24):7096-7122. doi: 10.1111/febs.15736. Epub 2021 Feb 16. FEBS J. 2021. PMID: 33513290 Free PMC article. Review.
-
Post-transcriptional methylation of mitochondrial-tRNA differentially contributes to mitochondrial pathology.Nat Commun. 2024 Oct 18;15(1):9008. doi: 10.1038/s41467-024-53318-x. Nat Commun. 2024. PMID: 39424798 Free PMC article.
-
Posttranscriptional modifications in mitochondrial tRNA and its implication in mitochondrial translation and disease.J Biochem. 2020 Nov 1;168(5):435-444. doi: 10.1093/jb/mvaa098. J Biochem. 2020. PMID: 32818253 Review.
-
Disease-associated mutations in mitochondrial precursor tRNAs affect binding, m1R9 methylation, and tRNA processing by mtRNase P.RNA. 2021 Apr;27(4):420-432. doi: 10.1261/rna.077198.120. Epub 2020 Dec 30. RNA. 2021. PMID: 33380464 Free PMC article.
-
Human mitochondrial diseases caused by lack of taurine modification in mitochondrial tRNAs.Wiley Interdiscip Rev RNA. 2011 May-Jun;2(3):376-86. doi: 10.1002/wrna.65. Epub 2011 Feb 25. Wiley Interdiscip Rev RNA. 2011. PMID: 21957023 Review.
References
-
- Ahmad RNR, Zhang LT, Morita R, Tani H, Wu Y, Chujo T, Ogawa A, Harada R, Shigeta Y, Tomizawa K, et al. 2024. Pathological mutations promote proteolysis of mitochondrial tRNA-specific 2-thiouridylase 1 (MTU1) via mitochondrial caseinolytic peptidase (CLPP). Nucleic Acids Res 52: 1341–1358. 10.1093/nar/gkad1197 - DOI - PMC - PubMed
-
- Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson NG, Stewart JB, Moraes CT. 2018. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24: 1696–1700. 10.1038/s41591-018-0166-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
