Thrombosis, Translational Medicine, and Biomarker Research: Moving the Needle
- PMID: 39719414
- PMCID: PMC12054438
- DOI: 10.1161/JAHA.124.038782
Thrombosis, Translational Medicine, and Biomarker Research: Moving the Needle
Abstract
Arterial and venous thromboembolism are leading causes of morbidity and death worldwide. Despite significant advances in the diagnosis, prognostication, and treatment of thrombotic diseases over the past 3 decades, the adoption of findings stemming from translational biomarker research in clinical practice remains limited. Biomarkers provide an opportunity to enhance our understanding of pathophysiological processes and optimize treatment strategies. They hold the promise of revolutionizing patient care. Still, this potential remains untapped, and several factors impede their use for near-patient applications. We sought to provide an overview of biomarker research in arterial and venous thromboembolic disease. We then aimed to discuss key barriers to the broader clinical implementation of biomarker research and highlight promising strategies to overcome them. We emphasize the merits of translational and implementation science to bridge the gaps from bench to bedside. Innovative trial design, data sharing, and collaborative efforts between academia and industry will be essential. Purposeful regression methodology using rational conceptual framework design, causal mediation analysis, and artificial intelligence might better leverage the use of observational data. Dedicated translational science training programs geared toward educating physicians on the appropriate measurement, interpretation, and integration of biomarker data in clinical practice should foster endorsement by frontline physicians. Finally, we make the case in support of a paradigm shift in cardiovascular medicine. Improved recognition of biomarker research and a greater emphasis on mechanistic evidence can better equip clinicians to deal with the uncertainty that defines the practice of thrombosis medicine.
Keywords: biomarkers; cardiovascular diseases; evidence‐based medicine; implementation science; thrombosis; translational science—biomedical.
Conflict of interest statement
Dr Shaw has received in‐kind laboratory support from Diagnostica Stago. Dr ten Cate has received research support from Bayer and consultancy fees from Astra Zeneca, Galapagos, Novostia, and Alveron; he is shareholder with CoagulationProfile, a Maastricht University spin‐off diagnostic company. All revenues are deposited at the CARIM Institute for Research. The remaining authors have no disclosures to report.
Figures
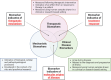

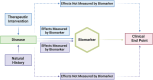
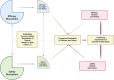


Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Venous Thromboembolism Research Priorities: A Scientific Statement From the American Heart Association and the International Society on Thrombosis and Haemostasis.Circulation. 2020 Aug 11;142(6):e85-e94. doi: 10.1161/CIR.0000000000000818. Epub 2020 Jul 8. Circulation. 2020. PMID: 32776842 Review.
-
Leveraging biomarkers and translational medicine for preclinical safety - Lessons for advancing the validation of alternatives to animal testing.ALTEX. 2024;41(4):545-566. doi: 10.14573/altex.2410011. ALTEX. 2024. PMID: 39440996
-
Translational precision medicine: an industry perspective.J Transl Med. 2021 Jun 5;19(1):245. doi: 10.1186/s12967-021-02910-6. J Transl Med. 2021. PMID: 34090480 Free PMC article. Review.
-
Collective intelligence for translational medicine: Crowdsourcing insights and innovation from an interdisciplinary biomedical research community.Ann Med. 2015;47(7):570-5. doi: 10.3109/07853890.2015.1091945. Epub 2015 Oct 15. Ann Med. 2015. PMID: 26469375 Review.
Cited by
-
Genome-wide association study-driven identification of thrombomodulin and factor V as the best biomarker combination for deep vein thrombosis.Genomics Inform. 2025 May 15;23(1):11. doi: 10.1186/s44342-025-00047-2. Genomics Inform. 2025. PMID: 40375318 Free PMC article.
References
-
- Jiménez D, de Miguel‐Díez J, Guijarro R, Trujillo‐Santos J, Otero R, Barba R, Muriel A, Meyer G, Yusen RD, Monreal M, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol. 2016;67:162–170. doi: 10.1016/j.jacc.2015.10.060 - DOI - PubMed
-
- Bertoletti L, Madridano O, Jiménez D, Muriel A, Bikdeli B, Ay C, Trujillo‐Santos J, Bosevski M, Sigüenza P, Monreal M. Cancer‐associated thrombosis: trends in clinical features, treatment, and outcomes from 2001 to 2020. JACC CardioOncol. 2023;5:758–772. doi: 10.1016/j.jaccao.2023.09.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

