Clinical Features and Current Pharmacotherapy of OAB in Practice: Ideal and Reality
- PMID: 39719461
- PMCID: PMC11787252
- DOI: 10.1007/s12325-024-03070-x
Clinical Features and Current Pharmacotherapy of OAB in Practice: Ideal and Reality
Abstract
Introduction: The present study aimed to investigate the prescribing patterns of anticholinergics (anti-AChR) or β3-adrenergic agonists (β3A) in the pharmacotherapy of overactive bladder (OAB) and to evaluate the differences in the frequency of adverse events (AEs) between the two types of drugs using a large-scale medical claims database.
Methods: This cohort study was conducted using the JMDC claims database between May 2015 and April 2023. Patient characteristics, prescription and treatment patterns of anti-AChR and β3A, and the incidence of AEs have been described.
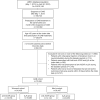
Results: Overall, 70,936 patients were analyzed [mean age, 53.6 (standard deviation: 12.3) years]. Among women (48.5%; 34,439), 21.4% initially received anti-AChR and 27.2% received β3A; among men (51.5%; 36,497), 17.1% initially received anti-AChR and 34.3% received β3A. Most patients (79.6%; women, 83.5%; men, 75.8%) visited clinics. About 10% of patients had a treatment change: 5.6% switched the drug type (change from anti-AChR to β3A or vice versa), and 4.0% had an add-on of another drug type. The incidence rate of treatment change per 100 patient-years was higher with β3A in both women (12.39) and men (13.65). In the multivariable analysis, initial prescription with anti-AChR compared with β3A did not show any association with the risk of AEs.
Conclusion: This large-scale database study revealed that treatment for OAB is often initiated with β3A and prescribed mainly at clinics. Changes or additions to initial prescriptions were as low as about 5%, indicating that raising awareness among physicians treating OAB is particularly important to improve the quality of life of patients with OAB. Our study also showed that the incidence of AEs was not associated with the initially prescribed drug type. Continued exploration is warranted to further clarify the risk of AEs with each prescription.
Keywords: Administrative database; Adrenergic β3 agonist; Anticholinergic; Claims database; Japan; Overactive bladder; Prescription; Retrospective study; Urinary tract infections.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Hiroshi Nakazawa, Takahiro Kitano, and Masashi Mikami were involved in research and preparation of the manuscript as employees of Pfizer Japan Inc. Kazuna Tsubouchi, Hisatomi Arima, Taiki Emoto, Chikao Aoyagi, Hiroshi Matsuzaki, Kosuke Tominaga, Naotaka Gunge, Takeshi Miyazaki, Yu Okabe, Nobuyuki Nakamura, Yuichiro Fukuhara, Masahiro Tachibana, Chizuru Nakagawa, Fumihiro Yamazaki, and Nobuhiro Haga did not receive any compensation related to this study from Pfizer Japan Inc. Ethical Approval: As we used the already anonymized JMDC dataset only, informed consent and ethical review/approval were not required for this study. The permission to access the JMDC database was under the license between Pfizer Japan Inc. and JMDC Inc.
Figures
References
-
- Takahashi S, Takei M, Asakura H, et al. Clinical guidelines for female lower urinary tract symptoms (second edition). Int J Urol. 2021;28:474–92. - PubMed
-
- AUA. AUA/SUFU Guideline. https://www.auanet.org/guidelines-and-quality/guidelines/overactive-blad.... Accessed Sep 30, 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous