Mpox mRNA-1769 vaccine inhibits orthopoxvirus replication at intranasal, intrarectal, and cutaneous sites of inoculation
- PMID: 39719481
- PMCID: PMC11668852
- DOI: 10.1038/s41541-024-01052-2
Mpox mRNA-1769 vaccine inhibits orthopoxvirus replication at intranasal, intrarectal, and cutaneous sites of inoculation
Abstract
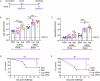
We previously reported that mice immunized twice with a lipid nanoparticle vaccine comprising four monkeypox viral mRNAs raised neutralizing antibodies and antigen-specific T cells and were protected against a lethal intranasal challenge with vaccinia virus (VACV). Here we demonstrated that the mRNA vaccine also protects mice against intranasal and intraperitoneal infections with monkeypox virus and bioluminescence imaging showed that vaccination greatly reduces or prevents VACV replication and spread from intranasal, rectal, and dermal inoculation sites. A single vaccination provided considerable protection that was enhanced by boosting for at least 4 months. Protection was related to the amount of mRNA inoculated, which correlated with neutralizing antibody levels. Furthermore, immunocompetent and immunodeficient mice lacking mature B and T cells that received serum from mRNA-immunized macaques before or after VACV challenge were protected. These findings provide insights into the mechanism and extent of mRNA vaccine-induced protection of orthopoxviruses and support clinical testing.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: TF, AC, and AWF are employees of Moderna and may hold stock/stock options in the company. All other authors declare no competing interests.
Figures







Update of
-
Mpox mRNA-1769 Vaccine Inhibits Orthopoxvirus Replication at Intranasal, Intrarectal, and Cutaneous Sites of Inoculation.bioRxiv [Preprint]. 2024 Sep 20:2024.09.19.613928. doi: 10.1101/2024.09.19.613928. bioRxiv. 2024. Update in: NPJ Vaccines. 2024 Dec 24;9(1):256. doi: 10.1038/s41541-024-01052-2. PMID: 39372733 Free PMC article. Updated. Preprint.
References
-
- Satheshkumar, P. S. & Damon, I. in Fields Virology: DNA Viruses, Vol. 4 (eds Howley, P. M., Knipe, D. M., Cohen, J. L., & Damania, B. A.) Ch. 17, 614–640 (Lippincott Williams & Wilkins, 2021).
-
- Fenner, F., Henderson, D. A., Arita, I., Jezek, Z. & Ladnyi, I. D. Smallpox and its Eradication. 1st edn (World Health Organization, 1988).
-
- Rao, A. K. et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices-United States, 2022. Morb. Mortal. Wkly. Rep.71, 734–742 (2022). - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

