Trimethylamine-N-oxide accelerates osteoporosis by PERK activation of ATF5 unfolding
- PMID: 39719538
- PMCID: PMC11668722
- DOI: 10.1007/s00018-024-05501-y
Trimethylamine-N-oxide accelerates osteoporosis by PERK activation of ATF5 unfolding
Abstract
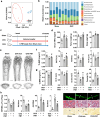
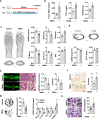
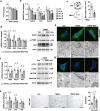
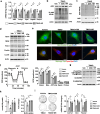
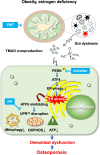
Imbalances in gut microbiota and their metabolites have been implicated in osteoporotic disorders. Trimethylamine-n-oxide (TMAO), a metabolite of L-carnitine produced by gut microorganisms and flavin-containing monooxygenase-3, is known to accelerate tissue metabolism and remodeling; however, its role in bone loss remained unexplored. This study investigates the relationship between gut microbiota dysbiosis, TMAO production, and osteoporosis development. We further demonstrate that the loss of beneficial gut microbiota is associated with the development of murine osteoporosis and alterations in the serum metabolome, particularly affecting L-carnitine metabolism. TMAO emerges as a functional metabolite detrimental to bone homeostasis. Notably, transplantation of mouse gut microbiota counteracts obesity- or estrogen deficiency-induced TMAO overproduction and mitigates key features of osteoporosis. Mechanistically, excessive TMAO intake augments bone mass loss by inhibiting bone mineral acquisition and osteogenic differentiation. TMAO activates the PERK and ATF4-dependent disruption of endoplasmic reticulum autophagy and suppresses the folding of ATF5, hindering mitochondrial unfolding protein response (UPRmt) in osteoblasts. Importantly, UPRmt activation by nicotinamide riboside mitigates TMAO-induced inhibition of mineralized matrix biosynthesis by preserving mitochondrial oxidative phosphorylation and mitophagy. Collectively, our findings revealed that gut microbiota dysbiosis leads to TMAO overproduction, impairing ER homeostasis and UPRmt, thereby aggravating osteoblast dysfunction and development of osteoporosis. Our study elucidates the catabolic role of gut microflora-derived TMAO in bone integrity and highlights the therapeutic potential of healthy donor gut microbiota transplantation to alter the progression of osteoporosis.
Keywords: ER-phagy; Gut microecosystem; Misfolding; OXPHOS; Parkin; Trimethylamine-n-oxide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Authors have no financial competing interest. Ethical approval and consent to participate: Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Kaohsiung Chang Gung Memorial Hospital (IACUC Affidavit #2019091103). This study did not involve human specimens or clinical data. Consent for publication: Not applicable.
Figures




References
-
- Lobo RA, Gompel A (2022) Management of menopause: a view towards prevention. Lancet Diabetes Endocrinol 10:457–470. 10.1016/S2213-8587(21)00269-2 - PubMed
-
- Seimon RV, Wild-Taylor AL, Keating SE et al (2019) Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO diet randomized clinical trial. JAMA Netw Open 2:e1913733. 10.1001/jamanetworkopen.2019.13733 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

