Doublecortin regulates the mitochondrial-dependent apoptosis in glioma via Rho-A/Net-1/p38-MAPK signaling
- PMID: 39719558
- PMCID: PMC11668075
- DOI: 10.1186/s10020-024-01021-4
Doublecortin regulates the mitochondrial-dependent apoptosis in glioma via Rho-A/Net-1/p38-MAPK signaling
Abstract
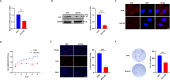
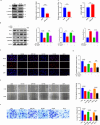
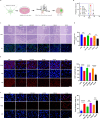
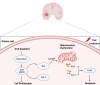
Doublecortin (DCX) is a microtubule-associated protein known to be a key regulator of neuronal migration and differentiation during brain development. However, the role of DCX, particularly in regulating the survival and growth of glioma cells, remains unclear. In this study, we utilized CRISPR/Cas9 technology to knock down DCX in the human glioma cell line (U251). DCX depletion suppressed cell proliferation and enhanced the pro-apoptotic effects of temozolomide (TMZ) and γ-radiation treatment. DCX knockdown led to the translocation of Bax to the mitochondria and mitochondria dysfunction. Furthermore, DCX deficiency-induced apoptosis took place along with the generation of reactive oxygen species (ROS), which is crucial in triggering mitochondrial membrane depolarization, the release of cytochrome c (Cyt-c), and caspase activation. Importantly, the transcriptional inhibition of DCX downregulated Rho-A, Net-1, and activated p38-MAPK cue, critical for cell survival and proliferation. Subsequent treatment with TMZ and γ-radiation further increased p38-MAPK activity through the decreased expression of Rho-A/Net-1, resulting in a significant reduction in glioma cell migration and invasion. Additionally, intracranial xenograft tumors of DCX-modified U251 cells in nude mice demonstrated inhibited tumor growth. Tumor sections treated with TMZ and γ-radiation exhibited a higher number of TUNEL-positive cells compared to the control group, indicating increased apoptosis. Our finding suggests that DCX depletion reduces glioma cell proliferation and promotes mitochondria-dependent apoptosis by enhancing the chemo and radiotherapy response. Targeting DCX represents a potential therapeutic target for glioma treatment.
Keywords: Apoptosis; CRISPR/Cas 9; Doublecortin; Glioma; Mitochondria.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study did not include human participants, data, or tissue. All animal experiments were compliant with the Xuzhou Medical University Ethics Committee. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Becker KN, Pettee KM, Sugrue A, Reinard KA, Schroeder JL, Eisenmann KM. The Cytoskeleton effectors rho-kinase (ROCK) and mammalian diaphanous-related (mDia) Formin have dynamic roles in Tumor Microtube formation in Invasive Glioblastoma cells. Cells. 2022;11:1559. 10.3390/cells11091559. - PMC - PubMed
-
- Breuzard G, Pagano A, Bastonero S, Malesinski S, Parat F, Barbier P, Peyrot V, Kovacic H. Tau regulates the microtubule-dependent migration of glioblastoma cells via the Rho-ROCK signaling pathway. J Cell Sci. 2019;132:jcs222851. 10.1242/jcs.222851. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

