Bronchoscopic biopsies - a novel source for primary airway epithelial cells in respiratory research
- PMID: 39719562
- PMCID: PMC11669235
- DOI: 10.1186/s12931-024-03060-1
Bronchoscopic biopsies - a novel source for primary airway epithelial cells in respiratory research
Abstract
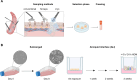
Background: Using primary airway epithelial cells (AEC) is essential to mimic more closely different types and stages of lung disease in humans while reducing or even replacing animal experiments. Access to lung tissue remains limited because these samples are generally obtained from patients who undergo lung transplantation for end-stage lung disease or thoracic surgery for (mostly) lung cancer. We investigated whether forceps or cryo biopsies are a viable alternative source of AEC compared to the conventional technique.
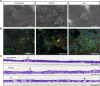
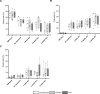
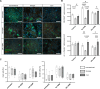
Methods: AECs were obtained ex vivo from healthy donor lung tissue using the conventional method and two biopsy procedures (forceps, cryo). The influence of the isolation method on the quality and function of AEC was investigated at different time-points during expansion and differentiation in air-liquid interface cultures. In addition, fully-differentiated AECs were stimulated with house dust mite extract (HDM) to allow functional analyses in an allergic in vitro model. Vitality or differentiation capacity were determined using flow cytometry, scanning electron microscope, periodic acid-Schiff reaction, immunofluorescence staining, and proteomics.
Results: As anticipated, no significant differences between each of the sampling methods were detected for any of the measured outcomes. The proteome composition was comparable for each isolation method, while donor-dependent effects were observed. Treatment with HDM led to minor differences in mucociliary differentiation.
Conclusions: Our findings confirmed the adequacy of these alternative approaches for attaining primary AECs, which can now expand the research for a broader range of lung diseases and for studies at an earlier stage not requiring lung surgery.
Keywords: 3R-principle; Air-liquid interface; Cryo biopsy; Forceps biopsy; In vitro disease-model; Primary airway epithelial cells.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The local ethics committee of the Medical Faculty of the University Duisburg-Essen as well as the Westdeutsche Biobank Essen approved the use, collection, and storage of lung transplant tissue (19-8717-BO,20-WBE-102) after informed written consent that was obtained from all patients or legal representatives involved in the study prior to tissue donation. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

