Consent to medical student teaching: an observational, cross-sectional study exploring the patient view
- PMID: 39719579
- PMCID: PMC11668103
- DOI: 10.1186/s12909-024-06557-x
Consent to medical student teaching: an observational, cross-sectional study exploring the patient view
Abstract
Background: New Zealand guidelines stipulate that patient consent is obtained for medical student involvement in clinical care, however, patients' preferences regarding consent for medical student teaching have not been widely explored. This study examined patient preferences for consent for medical student teaching with the aim to increase patient empowerment, to optimise care and to reflect societal expectations more accurately.
Method: Observational, semi-qualitative, cross-sectional study of in-patients. Each participant was presented with a series of nine hypothetical clinical scenarios and were allowed a limited number of responses. For each scenario the participants completed a short questionnaire about their preferences for consent. These included their preferred mode of consent (implicit, verbal or written), timing of consent, and who should take their consent. The analysis used descriptive statistics and ordinal logistic regression mixed models to investigate associations between patient characteristics and chosen mode of consent.
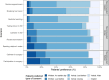
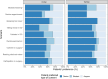
Results: There were 123 participants (50% male), median age was 64 years. Patients were admitted to either medical (69%), surgical (22%) or women's health (9%). Increasing age was statistically significantly associated with a preference for verbal and implicit rather than written consent with the exception of 'breaking bad news' and 'bedside teaching'. The majority of patients preferred verbal consent across all nine clinical scenarios (57-82%), including two surgical scenarios where verbal consent was preferred by 59%. Most patients preferred the supervising doctor to take consent, with no clear preference about the timing.
Conclusions: This study identifies the patient voice in the consent process for the involvement of medical students in clinical care. Although the patients' views generally align with an existing national consensus statement, there is variability in the expectations of the patients suggesting flexibility in the consent process is still needed. The preference for older patients for verbal or implicit consent compared with younger patients for more invasive scenarios highlights the need for consideration of inter-generational differences. Most patients in this study were willing to contribute to student learning in all scenarios.
Keywords: Informed consent; Medical student; Patients’ preferences; Patients’ view on consent; Patients’ view on medical student teaching; Sensitive examinations.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The patients were all older than 18 years of age and provided written informed consent to voluntarily participate in the study. Ethics approval was given by the University of Otago Ethics Committee (reference number H19/142). The study adhered to the “Declaration of Helsinki” set of ethical principles for medical research that involves human participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Involving Medical Students in Informed Consent: A Pilot Study.World J Surg. 2015 Sep;39(9):2214-9. doi: 10.1007/s00268-015-3090-9. World J Surg. 2015. PMID: 25956499
-
Understanding preferences regarding consent for pragmatic trials in acute care.Clin Trials. 2018 Dec;15(6):567-578. doi: 10.1177/1740774518801007. Epub 2018 Oct 3. Clin Trials. 2018. PMID: 30280582 Free PMC article.
-
Patients' beliefs regarding informed consent for low-risk pragmatic trials.BMC Med Res Methodol. 2017 Sep 18;17(1):145. doi: 10.1186/s12874-017-0424-3. BMC Med Res Methodol. 2017. PMID: 28923007 Free PMC article.
-
Teaching pelvic examinations under anaesthesia: what do women think?J Obstet Gynaecol Can. 2010 Jan;32(1):49-53. doi: 10.1016/S1701-2163(16)34404-8. J Obstet Gynaecol Can. 2010. PMID: 20370981
-
[Communication preferences of patients with prostate cancer : Preferences regarding the communication of bad news of patients with prostate cancer in Germany-results of an anonymous patient survey].Urologe A. 2016 Oct;55(10):1339-1346. doi: 10.1007/s00120-016-0154-x. Urologe A. 2016. PMID: 27306355 Review. German.
Cited by
-
Policy implications of physicians' attitudes towards being examined by medical students.Isr J Health Policy Res. 2025 Aug 13;14(1):50. doi: 10.1186/s13584-025-00711-6. Isr J Health Policy Res. 2025. PMID: 40797271 Free PMC article.
References
-
- Health and Disability Commissioner (Code of Health and Disability Services Consumers' Rights) Regulations 1996. Right 7. Available from: https://www.hdc.org.nz/your-rights/about-the-code/code-of-health-and-dis.... Accessed 18 July 2024.
-
- Code of Professional Conduct for Medical Students at the Universities of Auckland and Otago. 2020. Available at: https://www.otago.ac.nz/oms/otago614506.pdf.
-
- Malpas P, Bagg W, Yielder J, Merry A. Medical students, sensitive examinations and patient consent: a qualitative review. NZMJ. 2018;131(1482):29–37. - PubMed
-
- Bagg W, Adams J, Anderson L, Malpas P, Pidgeon G, Thorn M, et al. Medical Students and informed consent: A consensus statement prepared by the Faculties of Medical and Health Science of the Universities of Auckland and Otago, Chief Medical Officers of District Health Boards, New Zealand Medical Students’ Association and the Medical Council of New Zealand. NZMJ. 2015;128(1414):27–35. - PubMed
-
- Walker S, Reid P, Anderson L, Bull S, Jonas M, Manning J, et al. Informed consent for medical student involvement in patient care: an updated consensus statement. NZMJ. 2023;136(1579):86–95. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

