Evaluation and comparison of efficacy and safety of tirzepatide, liraglutide and SGLT2i in patients with type 2 diabetes mellitus: a network meta-analysis
- PMID: 39719583
- PMCID: PMC11668020
- DOI: 10.1186/s12902-024-01805-z
Evaluation and comparison of efficacy and safety of tirzepatide, liraglutide and SGLT2i in patients with type 2 diabetes mellitus: a network meta-analysis
Abstract
Objective: The objective is to assess the effectiveness and safety of tirzepatide, liraglutide, and SGLT2i in individuals diagnosed with type 2 diabetes.
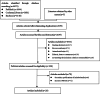
Methods: An inquiry was undertaken within the electronic database spanning from its inception to February 11th, 2024, aimed at identifying randomized controlled trials that assess the efficacy and safety of tirzepatide, liraglutide, canagliflozin, ertugliflozin, empagliflozin, dapagliflozin, and henagliflozin. Perform a network meta-analysis to examine the distinctions among them (PROSPERO registration number: CRD42024537006).
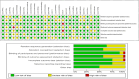
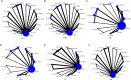
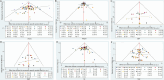
Results: Twenty-eight RCTs were included, involving 8499 participants. Compared with placebo, all treatments improved HbA1c levels: tirzepatide 15 mg reduced HbA1c the most (MD [95% CI], -2.24% [-2.52, -1.96]%), followed by tirzepatide 10 mg (MD [95% CI], -1.99% [-2.29, -1.69]%), tirzepatide 5 mg (MD [95% CI], -1.82% [-2.11, -1.53]%), and liraglutide 1.2 mg (MD [95% CI], -1.23% [-1.41, -1.05]%). Canagliflozin 300 mg also showed a significant reduction in HbA1c (MD [95% CI], -1.00% [-1.18, -0.82]). Tirzepatide was also the most effective in promoting weight loss, with the following results compared with placebo: tirzepatide 15 mg (MD [95% CI], -8.74 kg [-9.83, -7.66] kg), tirzepatide 10 mg (MD [95% CI], -7.13 kg [-8.40, -5.88] kg), tirzepatide 5 mg (MD [95% CI], -5.38 kg [-6.65, -4.11] kg), canagliflozin 300 mg (MD [95% CI], -2.31 kg [-2.79, -1.83] kg), and empagliflozin 10 mg (MD [95% CI], -2.00 kg [-2.44, -1.55] kg). In reducing systolic blood pressure (SBP), canagliflozin 300 mg showed the greatest effect (MD [95% CI], -5.96% [-7.96, -3.96] %). For diastolic blood pressure (DBP), henagliflozin 5 mg demonstrated the most significant reduction compared to placebo (MD [95% CI], -2.46% [-3.82, -1.10] %). Liraglutide 1.8 mg was most likely to cause adverse events (AE) (OR [95% CI], 2.57 [1.78, 3.70]), but there was no significant difference in serious adverse events (SAEs) between the interventions (including placebo).
Conclusion: Out of the seven medications examined in this study, tirzepatide demonstrates the most effective antidiabetic and weight-reducing effects. Furthermore, the dosage of Liraglutide at 1.2 mg and above demonstrates a more pronounced hypoglycemic effect in comparison to SGLT2 inhibitors. SGLT2 inhibitors exhibit a distinct hypotensive effect and are suitable for diabetic individuals experiencing hypertension.
Keywords: Liraglutide; Meta-analysis; SGLT2i; Tirzepatide; Type 2 diabetes mellitus.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This is a systematic review and meta-analysis, ethics approval and consent to participate are not applicable. Consent for publication: Not applicable. This study does not involve human participants. Competing interests: The authors declare no competing interests.
Figures
References
-
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. 10.1016/j.diabres.2021.109119. Epub 2021. Erratum in: Diabetes Res Clin Pract. 2023;204:110945.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical