Generation of vascularized pancreatic progenitors through co-differentiation of endoderm and mesoderm from human pluripotent stem cells
- PMID: 39719603
- PMCID: PMC11669215
- DOI: 10.1186/s13287-024-04120-5
Generation of vascularized pancreatic progenitors through co-differentiation of endoderm and mesoderm from human pluripotent stem cells
Abstract
Background: The simultaneous differentiation of human pluripotent stem cells (hPSCs) into both endodermal and mesodermal lineages is crucial for developing complex, vascularized tissues, yet poses significant challenges. This study explores a method for co-differentiation of mesoderm and endoderm, and their subsequent differentiation into pancreatic progenitors (PP) with endothelial cells (EC).
Methods: Two hPSC lines were utilized. By manipulating WNT signaling, we optimized co-differentiation protocols of mesoderm and endoderm through adjusting the concentrations of CHIR99021 and mTeSR1. Subsequently, mesoderm and endoderm were differentiated into vascularized pancreatic progenitors (vPP) by adding VEGFA. The differentiation characteristics and potential of vPPs were analyzed via transcriptome sequencing and functional assays.
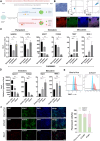
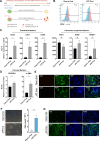
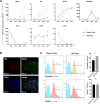
Results: A low-dose CHIR99021 in combination with mTeSR1 yielded approximately 30% mesodermal and 70% endodermal cells. Introduction of VEGFA significantly enhanced EC differentiation without compromising PP formation, increasing the EC proportion to 13.9%. Transcriptomic analyses confirmed the effectiveness of our protocol, showing up-regulation of mesodermal and endothelial markers, alongside enhanced metabolic pathways. Functional assays demonstrated that vPPs could efficiently differentiate into insulin-producing β-cells, as evidenced by increased expression of β-cell markers and insulin secretion.
Conclusion: Our findings provide a robust method for generating vPPs, which holds significant promise for regenerative medicine applications, particularly in diabetes treatment.
Keywords: Endoderm differentiation; Human pluripotent stem cells; Mesoderm differentiation; Multi-lineage co-differentiation; Vascularized pancreatic progenitors.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This study does not involve human participants or animals. Use of human pluripotent cell lines (UC and H1) was approved by the ethics committee of Shenzhen Hospital, Beijing University of Chinese Medicine in March 2021 (SZLDH2021LSYA-010). Consent for publication: Not applicable. Artificial intelligence: The authors declare that they have not use AI-generated work in this manuscript. Competing interests: The authors have declared no competing interests.
Figures



Similar articles
-
Generation of pancreatic progenitors from human pluripotent stem cells by small molecules.Stem Cell Reports. 2021 Sep 14;16(9):2395-2409. doi: 10.1016/j.stemcr.2021.07.021. Epub 2021 Aug 26. Stem Cell Reports. 2021. PMID: 34450037 Free PMC article.
-
Developing a Cost-Effective and Scalable Production of Human Hepatic Competent Endoderm from Size-Controlled Pluripotent Stem Cell Aggregates.Stem Cells Dev. 2018 Feb 15;27(4):262-274. doi: 10.1089/scd.2017.0074. Epub 2018 Feb 1. Stem Cells Dev. 2018. PMID: 29298619
-
Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1.Stem Cell Res Ther. 2018 Jan 23;9(1):15. doi: 10.1186/s13287-017-0759-z. Stem Cell Res Ther. 2018. PMID: 29361979 Free PMC article.
-
Conversion of embryonic stem cells into pancreatic beta-cell surrogates guided by ontogeny.Regen Med. 2006 May;1(3):327-36. doi: 10.2217/17460751.1.3.327. Regen Med. 2006. PMID: 17465786 Review.
-
Generating cells of the gastrointestinal system: current approaches and applications for the differentiation of human pluripotent stem cells.J Mol Med (Berl). 2012 Jul;90(7):763-71. doi: 10.1007/s00109-012-0923-y. Epub 2012 Jun 20. J Mol Med (Berl). 2012. PMID: 22714642 Review.
Cited by
-
Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting.Cells. 2025 May 1;14(9):665. doi: 10.3390/cells14090665. Cells. 2025. PMID: 40358189 Free PMC article. Review.
References
-
- Yamanaka S. Pluripotent stem cell-based Cell Therapy-Promise and challenges. Cell Stem Cell. 2020;27:523–31. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. - PubMed
-
- Wang S, Du Y, Zhang B, Meng G, Liu Z, Liew SY, et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell. 2024;187:6152–64. - PubMed
MeSH terms
Substances
Grants and funding
- JCYJ20220818103407016/Shenzhen Science and Technology Program
- SMRF.D2301015/Shenzhen Medical Research Fund
- 82172107/National Natural Science Foundation of China
- 2024A1515011222/Guangdong Basic and Applied Basic Research Foundation
- F-2022-Z99-502266/Special Funds for Strategic Emerging Industry of Shenzhen
LinkOut - more resources
Full Text Sources
Miscellaneous

