CTSS contributes to airway neutrophilic inflammation in mixed granulocytic asthma
- PMID: 39719614
- PMCID: PMC11669210
- DOI: 10.1186/s12931-024-03077-6
CTSS contributes to airway neutrophilic inflammation in mixed granulocytic asthma
Abstract
Background: Mixed granulocytic asthma (MGA) is usually associated with poor response to corticosteroid therapy and a high risk of severe asthma. Cathepsin S (CTSS) has been found to play an important role in various inflammatory diseases. This study was aimed to investigate the role of CTSS in MGA.
Methods: Induced sputum was obtained from healthy subjects and asthma patients. Two murine models of MGA were established using either TDI (toluene diisocyanate) alone or OVA emulsified in CFA. LY3000328, a specific antagonist of CTSS, was therapeutically given to BALB/c mice after airway challenge with TDI or OVA. The effects of recombinant CTSS was tested in vivo, and Akt inhibition was used to explore a possible mechanism for CTSS-induced airway inflammation.
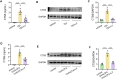
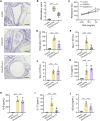
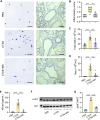
Results: MGA patients have a significant higher sputum CTSS level than the health and subjects with other inflammatory phenotypes, which was positively correlated with sputum level of soluble E-cadherin (sE-cadherin), sputum neutrophils, FeNO, FEF25-75% and glucocorticoid dosage. Allergen exposure markedly increased CTSS level and pharmacological antagonism of CTSS with LY3000328 decreased airway hyperresponsiveness, airway neutrophil accumulation, as well as the release of IL-17 and sE-cadherin in murine models of MGA, yet had no effects on eosinophilic inflammation nor type 2 inflammatory cytokines (IL-4 and IL-5). In addition, intratracheal instillation of recombinant CTSS leads to neutrophil recruitment and overproduction of sE-cadherin in the lung tissues, which could be attenuated by inhibition of Akt signaling.
Conclusion: Our data suggested that CTSS contributes to airway neutrophilic inflammation in MGA through an Akt-dependent pathway.
Keywords: Akt; CTSS; Mixed granulocytic asthma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All subjects signed informed consent forms approved by the Ethics Committee of our institution [Medical Ethics Year 2022 (No. 55)]. All animal experiments described here complied with the guidelines of the Committee of Guangzhou Medical University on the use and care of animals and were approved by the Animal Subjects Committee of Guangzhou Medical University. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Hamilton D, Lehman H. Asthma phenotypes as a guide for current and future biologic therapies. Clin Rev Allergy Immunol. 2020;59(2):160–74. - PubMed
-
- Gans MD, Gavrilova T. Understanding the immunology of asthma: pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr Respir Rev. 2020;Nov:36:118–27. - PubMed
-
- Agache I. Severe asthma phenotypes and endotypes. Semin Immunol. 2019;Dec:46:101301. - PubMed
-
- van Buul AR, Taube C. Treatment of severe asthma: entering the era of targeted therapy. Expert Opin Biol Ther. 2015;15(12):1713–25. - PubMed
-
- Tang H, Guo Y, Gan S, Chen Z, Dong M, Lin L, Chen H, Ji X, Xian M, Shi X, Tao A, Lv Y, Yao L, Chen R, Li S, Li J. GLUT1 mediates the release of HMGB1 from airway epithelial cells in mixed granulocytic asthma. Biochim Biophys Acta Mol Basis Dis. 2024;1870(3):167040. - PubMed
MeSH terms
Grants and funding
- 82161138020, U1801286/National Natural Science Foundation of China
- 82161138020, U1801286/National Natural Science Foundation of China
- 202102010011/Science and Technology Program of Guangzhou
- 202102010011/Science and Technology Program of Guangzhou
- ZNSA-2020003, ZNSA-2020013/Zhongnanshan Medical Foundation of Guangdong Province
- ZNSA-2020003, ZNSA-2020013/Zhongnanshan Medical Foundation of Guangdong Province
- 2022A1515012534/Natural Science Foundation of Guangdong Province
- 2022A1515012534/Natural Science Foundation of Guangdong Province
- 202201020370/Guangzhou Science and Technology Plan City-School Joint Funding Project
- 202201020370/Guangzhou Science and Technology Plan City-School Joint Funding Project
- SKLRD-Z-202325/the grant of State Key Laboratory of Respiratory Disease
- SKLRD-Z-202325/the grant of State Key Laboratory of Respiratory Disease
- 2021KCXTD028/Innovation team of respiratory diseases and regenerative medicine
- 2021KCXTD028/Innovation team of respiratory diseases and regenerative medicine
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

