STEAP4 with copper reductase activity suppresses tumorigenesis by regulating the cell cycle in hepatocellular carcinoma cells
- PMID: 39719623
- PMCID: PMC11669227
- DOI: 10.1186/s13008-024-00140-y
STEAP4 with copper reductase activity suppresses tumorigenesis by regulating the cell cycle in hepatocellular carcinoma cells
Abstract
Background: Abnormal expression of six-transmembrane epithelial antigen of prostate 4 (STEAP4) has been implicated in the carcinogenesis of hepatocellular carcinoma (HCC). However, the biological role and regulatory mechanisms of STEAP4 in HCC remain unclear.
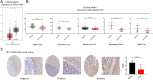
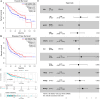
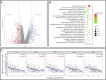
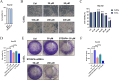
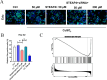
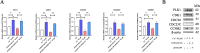
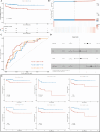
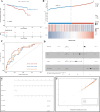
Methods and results: Here, we analyzed STEAP4 expression levels and differentially expressed genes (DEGs) between STEAP4 high- and low-expression groups using multiple databases. Proliferation assays, 5-ethynyl-2'-deoxyuridine (EdU) assays, propidium iodide (PI) flow cytometry, and colony formation assays were conducted to assess the effects of STEAP4 on HCC cell proliferation, cell cycle progression, and clonogenic capacity. STEAP4 was downregulated in HCC tumor tissues, with lower expression associated with poorer overall survival (OS) and disease-free survival (DFS) in patients. Functional network analysis suggested that STEAP4 regulates cell cycle signaling, with tumor sections showing a negative correlation between STEAP4 and cell cycle proteins. Overexpression of STEAP4, combined with non-cytotoxic copper exposure in the HepG2 cell line, reduced proliferation and clonogenicity, induced cell cycle arrest, and downregulated the mRNA and protein levels of cell cycle-regulating genes. A predictive model based on STEAP4 and cell cycle gene demonstrated prognostic value in HCC patients.
Conclusions: Our results lay a foundation for further study of the cell cycle regulatory role of STEAP4 with Cu2+ reductase activity in HCC, indicating that STEAP4 may be a promising therapeutic target for HCC.
Keywords: Cell cycle; Copper reductase activity; Hepatocellular carcinoma; STEAP4; Tumorigenesis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures in this study were conducted in accordance with the Ethics Committee of Third Affiliated Hospital of Sun Yat-sen University. Sample section staining related to this study were conducted in accordance with Declaration of Helsinki, and with the Ethics review board of Third Affiliated Hospital of Sun Yat-sen University with ethics approval number (II 2024-196). Consent was obtained from the participant. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Six-Transmembrane Epithelial Antigen of Prostate 4: An Indicator of Prognosis and Tumor Immunity in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2023 Apr 20;10:643-658. doi: 10.2147/JHC.S394973. eCollection 2023. J Hepatocell Carcinoma. 2023. PMID: 37101765 Free PMC article.
-
STEAP4 promoter methylation correlates with tumorigenesis of hepatocellular carcinoma.Pathol Res Pract. 2022 May;233:153870. doi: 10.1016/j.prp.2022.153870. Epub 2022 Apr 1. Pathol Res Pract. 2022. PMID: 35390632
-
STEAP4 inhibits cisplatin-induced chemotherapy resistance through suppressing PI3K/AKT in hepatocellular carcinoma.Cancer Metab. 2023 Dec 18;11(1):26. doi: 10.1186/s40170-023-00323-1. Cancer Metab. 2023. PMID: 38111065 Free PMC article.
-
STEAP4 modulates cell proliferation and oxidative stress in benign prostatic hyperplasia.Cell Signal. 2024 Jan;113:110933. doi: 10.1016/j.cellsig.2023.110933. Epub 2023 Oct 20. Cell Signal. 2024. PMID: 37866665
-
The Usefulness of STEAP Proteins in Prostate Cancer Clinical Practice.In: Bott SRJ, Ng KL, editors. Prostate Cancer [Internet]. Brisbane (AU): Exon Publications; 2021 May 27. Chapter 10. In: Bott SRJ, Ng KL, editors. Prostate Cancer [Internet]. Brisbane (AU): Exon Publications; 2021 May 27. Chapter 10. PMID: 34181381 Free Books & Documents. Review.
References
-
- Yamada N, Yasui K, Dohi O, Gen Y, Tomie A, Kitaichi T, Iwai N, Mitsuyoshi H, Sumida Y, Moriguchi M, Yamaguchi K, Nishikawa T, Umemura A, Naito Y, Tanaka S, Arii S, Itoh Y. Genome-wide DNA methylation analysis in hepatocellular carcinoma. Oncol Rep. 2016;35:2228–36. - PubMed
-
- Li PL, Liu H, Chen GP, Li L, Shi HJ, Nie HY, Liu Z, Hu YF, Yang J, Zhang P, Zhang XJ, She ZG, Li H, Huang Z, Zhu L. STEAP3 (six-transmembrane epithelial antigen of prostate 3) inhibits pathological cardiac hypertrophy. Hypertension. 2020;76:1219–30. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

