Multisystem clinicopathologic and genetic analysis of MELAS
- PMID: 39719631
- PMCID: PMC11668008
- DOI: 10.1186/s13023-024-03511-4
Multisystem clinicopathologic and genetic analysis of MELAS
Abstract
Background and objectives: Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) syndrome is a maternally inherited mitochondrial disorder that mostly affects the central nervous system and skeletal muscle. This study provides a comprehensive summary of the clinical symptoms, multisystemic pathogenesis, and genetic characteristics of MELAS syndrome. The aim was to improve comprehension of clinical practice and gain a deeper understanding of the latest pathophysiological theories.
Methods: The present investigation involved a cohort of patients diagnosed with MELAS at Nanjing Drum Tower Hospital between January 2014 and December 2022. Multisystem symptoms, magnetic resonance imaging/spectroscopy (MRI/MRS), muscle biopsy, and mitochondrial DNA (mtDNA) data were summarized and subsequently analysed.
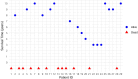
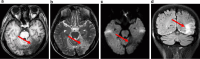
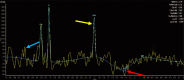
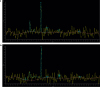
Results: This retrospective study included a cohort of 29 MELAS patients who predominantly presented symptoms such as stroke-like episodes, proximal muscle weakness, and exercise intolerance. MRI scans revealed very small infarcts beneath the deep cortex during stroke-like episodes, indicating nonvascular brain damage. Pathology analyses of the brain also showed neuronal degeneration and glial cell proliferation in the cerebral parenchyma. Proton magnetic resonance spectroscopy (1H-MRS) analysis revealed an increase in the lactate peak and a reduction in the N-acetylaspartate (NAA) level. Similarly, the phosphorus magnetic resonance spectroscopy (31P-MRS) analysis revealed an abnormal ratio of inorganic phosphate (Pi) to phosphocreatine (PCr). Muscle biopsy revealed the presence of ragged red fibres (RRFs) and cytochrome c oxidase (COX) enzyme-defective cells. These abnormalities indicate structural abnormalities in the mitochondria and deficiencies in oxidative phosphorylation, respectively. In addition to the common m.3243A > G variant, other prevalent variants, including m.5628 T > C, m.6352-13952del, and a 9-bp small deletion combined with m.3243A > G, exist.
Conclusions: MELAS is a rare mitochondrial syndrome characterized by clinical heterogeneity and genetic heteroplasmy. Abnormalities in mitochondrial metabolic function and impairments in enzyme activity are the pathogenic processes underlying MELAS. Mitochondrial vasculopathy and mitochondrial neuropathy may provide a partial explanation for the unique aetiology of stroke-like episodes.
Keywords: COX enzyme defects; MELAS; MRS; RRFs; Stroke-like episodes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures followed Chinese legal regulations and this study was approved by the Medical Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medicine School, under approval number 2020-349-03. All participants provided written informed consent and the procedures used in this study adhere to the tenets of the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors have no competing interests to declare that are relevant to the content of this article.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

