Mortality reduction with 23-valent pneumococcal polysaccharide vaccine: a systematic review and meta-analysis
- PMID: 39719643
- PMCID: PMC11669219
- DOI: 10.1186/s41479-024-00149-5
Mortality reduction with 23-valent pneumococcal polysaccharide vaccine: a systematic review and meta-analysis
Abstract
Background: Pneumococcal disease, caused by Streptococcus pneumoniae, imposes a significant global health burden, particularly affecting vulnerable groups such as the elderly and immunocompromised. The 23-valent pneumococcal polysaccharide vaccine (PPV23) is designed to protect against 23 serotypes of Streptococcus pneumoniae. However, there is ongoing debate about its effectiveness in reducing all-cause mortality. This systematic review and meta-analysis aimed to evaluate the efficacy of PPV23 in reducing all-cause and pneumonia-related mortality among adults.
Methods: A systematic search was conducted across PubMed, Embase, and Web of Science, focusing on studies that evaluated the mortality outcomes of adults vaccinated with PPV23 compared to non-vaccinated adults. Both randomized controlled trials (RCTs) and observational studies were included, while case reports, case series, and non-human studies were excluded. Data extraction and quality assessment were facilitated by Nested Knowledge software, using the Newcastle-Ottawa Scale for observational studies and the Cochrane Risk of Bias tool for RCTs.
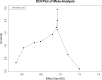
Results: The search yielded 826 records, with 19 studies meeting the inclusion criteria. The pooled analysis of four RCTs showed no significant reduction in all-cause mortality (RR = 1.030; 95% CI: 0.945, 1.122). However, analysis of pneumonia-related mortality across various studies indicated a significant reduction (HR = 0.504; 95% CI: 0.316, 0.693). Moderate to high heterogeneity was noted in mortality studies, and a potential publication bias was identified.
Conclusion: The findings suggest that while PPV23 may not significantly reduce all-cause mortality, it is effective in reducing pneumonia-related mortality among adults, particularly in those at higher risk. These results support the continued use of PPV23 in targeted adult populations, emphasizing the need for more primary studies to explore its effectiveness across diverse groups.
Keywords: Meta-analysis; Mortality; PPV23; Pneumonia; Vaccination.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not required. Competing interests: The authors declare no competing interests.
Figures



References
-
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health. 2018;6(7):e744–57. - DOI - PMC - PubMed
-
- Kumar P, Neyazi A, Rillera Marzo R, Augusto Guimaraes C, Barboza J, Abu Serhan J. H, Mycoplasma pneumoniae returns: understanding its spread and growing impact. Evid. 2024;2(1).
-
- Savoy M, Pneumococcal Vaccine MSD, Manual Professional, Edition. MSD Manual; 2024 [updated 2024/04. https://www.msdmanuals.com/en-in/professional/infectious-diseases/immuni...
Publication types
LinkOut - more resources
Full Text Sources

