Dysregulation of Myelination in Focal Cortical Dysplasia Type II of the Human Frontal Lobe
- PMID: 39719691
- PMCID: PMC11920682
- DOI: 10.1002/glia.24662
Dysregulation of Myelination in Focal Cortical Dysplasia Type II of the Human Frontal Lobe
Abstract
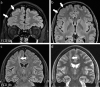
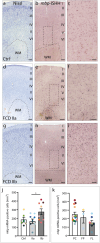
Focal cortical dysplasias (FCDs) are local malformations of the human neocortex and a leading cause of intractable epilepsy. FCDs are classified into different subtypes including FCD IIa and IIb, characterized by a blurred gray-white matter boundary or a transmantle sign indicating abnormal white matter myelination. Recently, we have shown that myelination is also compromised in the gray matter of FCD IIa of the temporal lobe. Since myelination is key for brain function, which is imbalanced in epilepsy, in the current study, we investigated myelination in the gray matter of FCD IIa and IIb from the frontal lobe on the morphological, ultrastructural, and transcriptional level. We found that FCD IIa presents with an ordinary radial myelin fiber pattern, but with a reduced thickness of myelin sheaths of 500-1000 nm thick axons in comparison to FCD IIb and with an attenuation of the myelin synthesis machinery. In contrast, FCD IIb showed an irregular and disorganized myelination pattern covering an enlarged area in comparison to FCD IIa and controls and with increased numbers of myelinating oligodendrocytes (OLs). FCD IIb had significantly thicker myelin sheaths of large caliber axons (above 1000 nm) when compared to FCD IIa. Accordingly, FCD IIb showed a significant up-regulation of myelin-associated mRNAs in comparison to FCD IIa and enhanced binding capacities of the transcription factor MYRF to target sites in myelin-associated genes. These data indicate that FCD IIa and IIb are characterized by a differential dysregulation of myelination in the gray matter of the frontal lobe.
Keywords: axon; chromatin; cortical malformation; epilepsy; neocortex; oligodendrocyte; transcriptional regulation.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Baumann, N. , and Pham‐Dinh D.. 2001. “Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System.” Physiological Reviews 81: 871–927. - PubMed
-
- Bernasconi, A. , Cendes F., Theodore W. H., et al. 2019. “Recommendations for the Use of Structural Magnetic Resonance Imaging in the Care of Patients With Epilepsy: A Consensus Report From the International League Against Epilepsy Neuroimaging Task Force.” Epilepsia 60: 1054–1068. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

