A neurodegenerative cellular stress response linked to dark microglia and toxic lipid secretion
- PMID: 39719704
- PMCID: PMC12481204
- DOI: 10.1016/j.neuron.2024.11.018
A neurodegenerative cellular stress response linked to dark microglia and toxic lipid secretion
Abstract
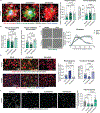
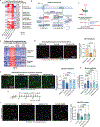
The brain's primary immune cells, microglia, are a leading causal cell type in Alzheimer's disease (AD). Yet, the mechanisms by which microglia can drive neurodegeneration remain unresolved. Here, we discover that a conserved stress signaling pathway, the integrated stress response (ISR), characterizes a microglia subset with neurodegenerative outcomes. Autonomous activation of ISR in microglia is sufficient to induce early features of the ultrastructurally distinct "dark microglia" linked to pathological synapse loss. In AD models, microglial ISR activation exacerbates neurodegenerative pathologies and synapse loss while its inhibition ameliorates them. Mechanistically, we present evidence that ISR activation promotes the secretion of toxic lipids by microglia, impairing neuron homeostasis and survival in vitro. Accordingly, pharmacological inhibition of ISR or lipid synthesis mitigates synapse loss in AD models. Our results demonstrate that microglial ISR activation represents a neurodegenerative phenotype, which may be sustained, at least in part, by the secretion of toxic lipids.
Keywords: Alzheimer’s disease; ISR; dark microglia; integrated stress response; lipid secretion; lipotoxicity; microglia; neurodegeneration; neurotoxic microglia; non-cell-autonomous stress.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Chen Y & Colonna M Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? Journal of Experimental Medicine 218, doi: 10.1084/jem.20202717 (2021). - DOI
-
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e1217, doi: 10.1016/j.cell.2017.05.018 (2017). - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

