Part 2: Drug Interactions Involving Cannabis Products in Persons Aged 18 and Over: A Summary of Published Case Reports and Analysis of the FDA Adverse Event Reporting System
- PMID: 39719832
- PMCID: PMC11668913
- DOI: 10.1002/prp2.70047
Part 2: Drug Interactions Involving Cannabis Products in Persons Aged 18 and Over: A Summary of Published Case Reports and Analysis of the FDA Adverse Event Reporting System
Abstract
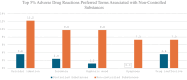
The increasing utilization of cannabis products combined with lack of data regarding potential cannabis-prescription drug interactions is concerning. This study aimed to review published case reports and FDA Adverse Event Reporting System (FAERS) spontaneous reports to assess cannabis-drug interactions in persons aged 18 and over. A literature search identified 20 case reports that were each assessed for drug interaction causality using the Drug Interaction Probability Scale. Data collected from the FAERS revealed a greater proportion of reports mentioning serious outcomes, including death, when cannabis was used concomitantly with controlled substances compared to noncontrolled substances. Fisher's exact test showed a statistically significant difference between the controlled and noncontrolled groups (p = 0.043). Overall, these findings emphasize the need for additional research and vigilant monitoring of cannabis use when combined with other medications.
© 2024 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
M.R.C., X.L., K.A., S.B.T., R.D.B., S.E.: has no conflict of interest or disclosures to report. S.L.K‐G. receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK121730 and U01DK130010, the National Center for Complementary and Integrative Health U54AT008909, and the Jewish Healthcare Foundation. M.F.P. receives grant funding from the National Center for Complementary and Integrative Health and Office of Dietary Supplements (U54AT008909), National Institute of Child Health and Human Development (R01HD081299), and National Institute of General Medical Sciences (R16GM146679), and MFP is a member of the Scientific Advisory Board for Simcyp, Certara UK Limited.
Figures


References
-
- “North America Legal Cannabis Market Size Report, 2030,” accessed December 19, 2022, https://www.grandviewresearch.com/industry‐analysis/north‐america‐legal‐....
-
- “Getting Medical Marijuana in Pennsylvania. PA.Gov,” accessed April 10, 2024, https://www.pa.gov/guides/pennsylvania‐medical‐marijuana‐program/.
-
- Bansal S., Maharao N., Paine M. F., and Unadkat J. D., “Predicting the Potential for Cannabinoids to Precipitate Pharmacokinetic Drug Interactions via Reversible Inhibition or Inactivation of Major Cytochromes P450,” Drug Metabolism and Disposition: The Biological Fate of Chemicals 48, no. 10 (2020): 1008–1017, 10.1124/dmd.120.000073. - DOI - PMC - PubMed
-
- Bansal S., Paine M. F., and Unadkat J. D., “Comprehensive Predictions of Cytochrome P450 (P450)‐mediated in Vivo Cannabinoid‐Drug Interactions Based on Reversible and Time‐Dependent P450 Inhibition in Human Liver Microsomes,” Drug Metabolism and Disposition: The Biological Fate of Chemicals 50, no. 4 (2022): 351–360, 10.1124/dmd.121.000734. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

