OCTN1 mediates acetylcholine transport in the A549 lung cancer cells: possible pathophysiological implications
- PMID: 39719963
- PMCID: PMC11666908
- DOI: 10.3389/fmolb.2024.1512530
OCTN1 mediates acetylcholine transport in the A549 lung cancer cells: possible pathophysiological implications
Abstract
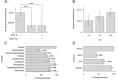
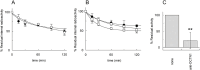
A role for acetylcholine in cell proliferation, epithelial mesenchymal transition and invasion has been well assessed and related to the presence of the non-neuronal cholinergic system in lung cancer. For the operation of this non-neuronal system, acetylcholine should be released by a transporter mediated non-quantal process. OCTN1 is one of the transporters able to catalyse acetylcholine efflux in vitro and ex vivo. Using the A549 cell line as a lung cancer model, it has been found that these cells express OCTN1 at a higher level with respect to other cancer cells. The transport capacity of OCTN1 extracted from A549 and reconstituted into proteoliposomes reflects the protein expression profile. The properties of the acetylcholine transport mediated by OCTN1 of A549 in terms of specificity to ligands and ability to catalyse efflux of acetylcholine correspond to those previously described for the same transporter in other cells or to those of the human recombinant protein. OCTN1 is the major player in acetylcholine release in A549 and, therefore, may represent a target for inhibitors able to block the acetylcholine action in this type of aggressive tumors.
Keywords: Octn1; SLC; drug discovery; lung cancer; non-neuronal cholinergic system.
Copyright © 2024 Pochini, Tedesco, Mazza, Scalise and Indiveri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Immuno-detection of OCTN1 (SLC22A4) in HeLa cells and characterization of transport function.Int Immunopharmacol. 2015 Nov;29(1):21-6. doi: 10.1016/j.intimp.2015.04.040. Epub 2015 Apr 29. Int Immunopharmacol. 2015. PMID: 25937167
-
Acetylcholine and acetylcarnitine transport in peritoneum: Role of the SLC22A4 (OCTN1) transporter.Biochim Biophys Acta. 2016 Apr;1858(4):653-60. doi: 10.1016/j.bbamem.2015.12.026. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26724204
-
The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn's disease.Biochim Biophys Acta. 2012 Mar;1818(3):559-65. doi: 10.1016/j.bbamem.2011.12.014. Epub 2011 Dec 21. Biochim Biophys Acta. 2012. PMID: 22206629
-
OCTN1: A Widely Studied but Still Enigmatic Organic Cation Transporter Linked to Human Pathology and Drug Interactions.Int J Mol Sci. 2022 Jan 14;23(2):914. doi: 10.3390/ijms23020914. Int J Mol Sci. 2022. PMID: 35055100 Free PMC article. Review.
-
Involvement of mammalian SoLute Carriers (SLC) in the traffic of polyamines.Front Mol Biosci. 2024 Jul 25;11:1452184. doi: 10.3389/fmolb.2024.1452184. eCollection 2024. Front Mol Biosci. 2024. PMID: 39130372 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

