Modulation of oxidative stress/NMDA/nitric oxide pathway by topiramate attenuates morphine dependence in mice
- PMID: 39719994
- PMCID: PMC11667026
- DOI: 10.1016/j.heliyon.2024.e40584
Modulation of oxidative stress/NMDA/nitric oxide pathway by topiramate attenuates morphine dependence in mice
Abstract
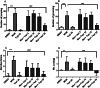
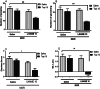
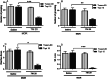
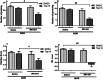
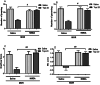
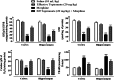
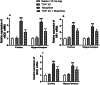
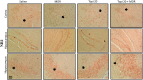
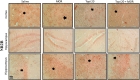
Morphine belongs to the class of opioids and is known for its potential to cause dependence and addiction, particularly with prolonged use. Due to the associated risks, caution must be taken when prescribing and limiting its clinical use. Overexpression of N-methyl-D-aspartate (NMDA) receptors, nitric oxide and cGMP pathway has been implicated in exacerbate the development of morphine dependence and withdrawal. Topiramate, an antiepileptic drug, interacts with various receptors, ion channels and certain enzymes. In this study, we investigated the effects of topiramate on morphine dependence in mice, specifically targeting NMDA/Nitric oxide/cGMP pathway. Mice were administered different doses of topiramate (intraperitoneally) during the development phase, 45 min prior to morphine administration. Topiramate (20 mg/kg) significantly reduced naloxone-induced withdrawal symptoms in morphine-dependent mice. Additionally, subeffective doses of topiramate, when co-administered with NMDA receptor antagonist MK-801 (0.05 mg/kg) or nitric oxide synthase inhibitors such as L-NAME (10 mg/kg, a non-specific NOS inhibitor) and 7-NI (20 mg/kg, a selective nNOS inhibitor), showed a marked reduction in withdrawal signs. However, the effect of topiramate (20 mg/kg) was abolished when co-administered with NMDA (75 mg/kg, an NMDA receptor agonist) or L-arginine (60 mg/kg, a NOS substrate). Ex-vivo analysis revealed that topiramate significantly reduced oxidative stress and downregulated the gene expression of nNOS, NR1, and NR2B in morphine-treated mice. Furthermore, the expression of NR1 and NR2B proteins in the hippocampus and cortex was significantly reduced in topiramate-pretreated mice. Hence, this finding suggest that topiramate mitigates morphine dependence and withdrawal by inhibiting oxidative stress and modulating the NMDA/NO pathway.
Keywords: Dependence; MK-801; Morphine; NMDA; Nitric oxide; Oxidative stress.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Muhammad Imran Khan reports financial support was provided by 10.13039/501100004681Higher Education Commission Pakistan. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




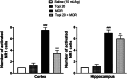

Similar articles
-
Involvement of NMDA receptors and L-arginine/nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effects of topiramate in mice forced swimming test.Brain Res Bull. 2016 Apr;122:62-70. doi: 10.1016/j.brainresbull.2016.03.004. Epub 2016 Mar 14. Brain Res Bull. 2016. PMID: 26988103
-
Spinal NMDA receptor--nitric oxide mediation of the expression of morphine withdrawal symptoms in the rat.Brain Res. 1995 May 15;679(2):189-99. doi: 10.1016/0006-8993(95)00203-3. Brain Res. 1995. PMID: 7633880
-
Interaction of morphine tolerance with pentylenetetrazole-induced seizure threshold in mice: The role of NMDA-receptor/NO pathway.Epilepsy Behav. 2020 Nov;112:107343. doi: 10.1016/j.yebeh.2020.107343. Epub 2020 Aug 2. Epilepsy Behav. 2020. PMID: 32755816
-
The effects of simultaneous administration of alpha(2) -adrenergic agents with L-NAME or L-arginine on the development and expression of morphine dependence in mice.Behav Pharmacol. 2002 Mar;13(2):117-25. doi: 10.1097/00008877-200203000-00003. Behav Pharmacol. 2002. PMID: 11981224
-
Opiate physical dependence and N-methyl-D-aspartate receptors.Eur J Pharmacol. 2004 Oct 1;500(1-3):121-8. doi: 10.1016/j.ejphar.2004.07.017. Eur J Pharmacol. 2004. PMID: 15464026 Review.
References
-
- Ahmadi S., et al. Repeated use of morphine induces anxiety by affecting a proinflammatory cytokine signaling pathway in the prefrontal cortex in rats. Mol. Neurobiol. 2023;60(3):1425–1439. https://10.1007/s12035-022-03144-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources

