Aromatic-aromatic interactions drive fold switch of GA95 and GB95 with three residue difference
- PMID: 39720130
- PMCID: PMC11665817
- DOI: 10.1039/d4sc04951a
Aromatic-aromatic interactions drive fold switch of GA95 and GB95 with three residue difference
Abstract
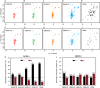
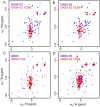
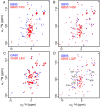
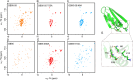
Proteins typically adopt a single fold to carry out their function, but metamorphic proteins, with multiple folding states, defy this norm. Deciphering the mechanism of conformational interconversion of metamorphic proteins is challenging. Herein, we employed nuclear magnetic resonance (NMR), circular dichroism (CD), and all-atom molecular dynamics (MD) simulations to elucidate the mechanism of fold switching in proteins GA95 and GB95, which share 95% sequence homology. The results reveal that long-range interactions, especially aromatic π-π interactions involving residues F52, Y45, F30, and Y29, are critical for the protein switching from a 3α to a 4β + α fold. This study contributes to understanding how proteins with highly similar sequences fold into distinct conformations and may provide valuable insights into the protein folding code.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Relative free enthalpies for point mutations in two proteins with highly similar sequences but different folds.Biochemistry. 2013 Jul 23;52(29):4962-70. doi: 10.1021/bi400272q. Epub 2013 Jul 11. Biochemistry. 2013. PMID: 23802564
-
Slow ring flips in aromatic cluster of GB1 studied by aromatic 13C relaxation dispersion methods.J Biomol NMR. 2020 Mar;74(2-3):183-191. doi: 10.1007/s10858-020-00303-3. Epub 2020 Feb 3. J Biomol NMR. 2020. PMID: 32016706 Free PMC article.
-
The role of aromatic residues in the hydrophobic core of the villin headpiece subdomain.Protein Sci. 2002 Mar;11(3):680-7. doi: 10.1110/ps.22202. Protein Sci. 2002. PMID: 11847290 Free PMC article.
-
Metamorphic Proteins: Emergence of Dual Protein Folds from One Primary Sequence.Biochemistry. 2017 Jun 20;56(24):2971-2984. doi: 10.1021/acs.biochem.7b00375. Epub 2017 Jun 12. Biochemistry. 2017. PMID: 28570055 Review.
-
Exploring the structural acrobatics of fold-switching proteins using simplified structure-based models.Biophys Rev. 2023 Jul 14;15(4):787-799. doi: 10.1007/s12551-023-01087-0. eCollection 2023 Aug. Biophys Rev. 2023. PMID: 37681096 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

