The modulating role of uniaxial straining in the IL-1β and TGF-β mediated inflammatory response of human primary ligamentocytes
- PMID: 39720167
- PMCID: PMC11666359
- DOI: 10.3389/fbioe.2024.1469238
The modulating role of uniaxial straining in the IL-1β and TGF-β mediated inflammatory response of human primary ligamentocytes
Abstract
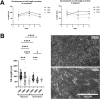
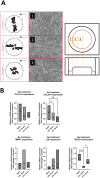
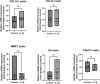
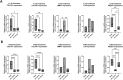
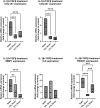
Biomechanical (over-)stimulation, in addition to inflammatory and fibrotic stimuli, severely impacts the anterior cruciate ligament (ACL) biology, contributing to the overall chronic nature of desmopathy. A major challenge has been the lack of representative two-dimensional (2D) in vitro models mimicking inflammatory processes in the presence of dynamic mechanical strain, both being crucial for ligament homeostasis. Physiological levels of strain exert anti-inflammatory effects, while excessive strain can facilitate inflammatory mechanisms. Adhering to the 3Rs (Replacement, Reduction and Refinement) principles of animal research, this study aims to investigate the role of a dynamic biomechanical in vitro environment on inflammatory mechanisms by combining a Flexcell culture system with primary human ligamentocytes for the study of ligament pathology. Primary ligamentocytes from OA patients were cultured under animal-free conditions with human platelet lysate, and exposed to either IL-1β or TGF-β3 to simulate different inflammatory microenvironments. Cells were subjected to different magnitudes of mechanical strain. Results showed that cells aligned along the force axis under strain. This study highlights the critical role of the mechanical microenvironment in modulating inflammatory and fibrotic cellular responses in ligamentocyte pathology, providing valuable insights into the complex interplay between biomechanical stimuli and cytokine signaling. These findings not only advance our understanding of ligament biology but also can pave the way for the development of more targeted therapeutic strategies for ligament injuries and diseases, potentially improving patient outcomes in orthopedic medicine.
Keywords: fibrosis; in vitro modelling; inflammation; ligamentocytes; mechanical loading.
Copyright © 2024 Heidenberger, Hangel, Reihs, Strauss, Liskova, Alphonsus, Brunner, Döring, Gerner, Jenner, Windhager, Toegel and Rothbauer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Altmann N., Bowlby C., Coughlin H., Belacic Z., Sullivan S., Durgam S. (2023). Interleukin-6 upregulates extracellular matrix gene expression and transforming growth factor β1 activity of tendon progenitor cells. BMC Musculoskelet. Disord. 24 (1), 907. 10.1186/S12891-023-07047-9 - DOI - PMC - PubMed
-
- Beaumont R. E., Smith E. J., Zhou L., Marr N., Thorpe C. T., Guest D. J. (2024). Exogenous interleukin-1 beta stimulation regulates equine tenocyte function and gene expression in three-dimensional culture which can be rescued by pharmacological inhibition of interleukin 1 receptor, but not nuclear factor kappa B, signaling. Mol. Cell. Biochem. 479 (5), 1059–1078. 10.1007/S11010-023-04779-Z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

