Ultrasmall Antioxidant Copper Nanozyme to Enhance Stem Cell Microenvironment for Promoting Diabetic Wound Healing
- PMID: 39720217
- PMCID: PMC11668326
- DOI: 10.2147/IJN.S487647
Ultrasmall Antioxidant Copper Nanozyme to Enhance Stem Cell Microenvironment for Promoting Diabetic Wound Healing
Abstract
Purpose: Stem cell therapy is a promising approach for treating chronic diabetic wounds. However, its effectiveness is significantly limited by the high oxidative stress environment and persistent inflammation induced by diabetes. Strategies to overcome these challenges are essential to enhance the therapeutic potential of stem cell therapy.
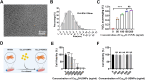
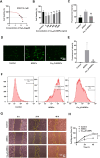
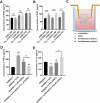
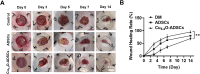
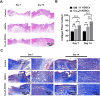
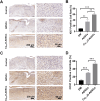
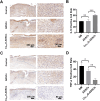
Methods: Cu5.4O ultrasmall nanoparticles (Cu5.4O-USNPs), known for their excellent reactive oxygen species (ROS) scavenging properties, were utilized to protect adipose-derived stem cells (ADSCs) from oxidative stress injury. In vitro experiments were conducted to evaluate the viability, paracrine activity, and anti-inflammatory capabilities of ADSCs loaded with Cu5.4O-USNPs under oxidative stress conditions. In vivo experiments in diabetic mice were performed to assess the therapeutic effects of Cu5.4O-USNP-loaded ADSCs on wound healing, including their impact on inflammation, collagen synthesis, angiogenesis, and wound closure.
Results: ADSCs treated with Cu5.4O-USNPs showed significantly enhanced viability, paracrine activity, and anti-inflammatory properties under oxidative stress conditions in vitro. In diabetic mice, Cu5.4O-USNP-loaded ADSCs reduced inflammatory responses in wound tissues, promoted collagen synthesis and angiogenesis, and accelerated diabetic wound healing. These findings suggest that Cu5.4O-USNPs effectively mitigate the adverse effects of oxidative stress and inflammation, enhancing the therapeutic efficacy of ADSCs.
Conclusion: This study presents a simple and effective approach to improve the therapeutic potential of stem cell therapy for diabetic wounds. By incorporating Cu5.4O-USNPs, the antioxidative and anti-inflammatory capabilities of ADSCs are significantly enhanced, offering a promising strategy for ROS-related tissue repair and chronic wound healing.
Keywords: ADSCs; Cu5.4O-USNPs; inflammatory environment; stem cell therapy.
© 2024 Hou et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Integrated cascade antioxidant nanozymes-Cu5.4O@CNDs combat acute liver injury by regulating retinol metabolism.Theranostics. 2025 Apr 21;15(12):5592-5615. doi: 10.7150/thno.106811. eCollection 2025. Theranostics. 2025. PMID: 40365282 Free PMC article.
-
Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases.Nat Commun. 2020 Jun 3;11(1):2788. doi: 10.1038/s41467-020-16544-7. Nat Commun. 2020. PMID: 32493916 Free PMC article.
-
A hybrid of ultrathin metal-organic framework sheet and ultrasmall copper nanoparticles for detection of hydrogen peroxide with enhanced activity.Anal Bioanal Chem. 2021 Jan;413(3):839-851. doi: 10.1007/s00216-020-03038-0. Epub 2020 Nov 21. Anal Bioanal Chem. 2021. PMID: 33219832
-
Adipose-derived stem cells: Effectiveness and advances in delivery in diabetic wound healing.Biomed Pharmacother. 2018 Nov;107:625-633. doi: 10.1016/j.biopha.2018.08.013. Epub 2018 Aug 14. Biomed Pharmacother. 2018. PMID: 30118878 Review.
-
Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential.Int Wound J. 2025 May;22(5):e70330. doi: 10.1111/iwj.70330. Int Wound J. 2025. PMID: 40288766 Free PMC article. Review.
Cited by
-
The Journey of Copper-Impregnated Dressings in Wound Healing: From a Medical Hypothesis to Clinical Practice.Biomedicines. 2025 Feb 24;13(3):562. doi: 10.3390/biomedicines13030562. Biomedicines. 2025. PMID: 40149539 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

