Novel enantiopure δ-thiolactones: synthesis, structural characterization, and reactivity studies
- PMID: 39720259
- PMCID: PMC11667218
- DOI: 10.1039/d4ra07780f
Novel enantiopure δ-thiolactones: synthesis, structural characterization, and reactivity studies
Abstract
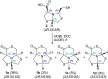
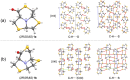
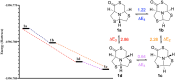
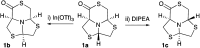
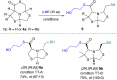
A new series of chiral δ-thiolactone derivatives have been prepared. These compounds exemplify the acetalic N-C-S reversibility of fused thiazolidines toward the thermodynamic product. The stereochemistry of the synthesized compounds was elucidated using X-ray crystallography, NOESY spectroscopy, and DFT calculations. The aminolysis reaction of the δ-thiolactone was studied with various alkyl amines, which can open the thioester to yield amido thiols in a single step. This reaction has the potential to be applied in the synthesis of bioactive compounds, polymer chemistry, and dynamic combinatorial chemistry, among others fields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


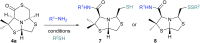

References
-
- Hirschbeck V. Gehrtz P. H. Fleischer I. Regioselective Thiocarbonylation of Vinyl Arenes. J. Am. Chem. Soc. 2016;138:16794–16799. - PubMed
-
- Pietrocola F. Galluzzi L. Bravo-San Pedro J. M. Madeo F. Kroemer G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015;21:805–821. - PubMed
-
- Agouridas V. El Mahdi O. Diemer V. Cargoët M. Monbaliu J. C. M. Melnyk O. Native Chemical Ligation and Extended Methods: Mechanisms, Catalysis, Scope, and Limitations. Chem. Rev. 2019;119:7328–7443. - PubMed
-
- Vallee Y. Shalayel I. Ly K. D. Rao K. V. R. De Paëpe G. Märker K. et al., At the very beginning of life on Earth: the thiol-rich peptide (TRP) world hypothesis. Int. J. Dev. Biol. 2017;61:471–478. - PubMed
LinkOut - more resources
Full Text Sources

