Genetic vulnerability and adverse mental health outcomes following mild traumatic brain injury: a meta-analysis of CENTER-TBI and TRACK-TBI cohorts
- PMID: 39720422
- PMCID: PMC11667043
- DOI: 10.1016/j.eclinm.2024.102956
Genetic vulnerability and adverse mental health outcomes following mild traumatic brain injury: a meta-analysis of CENTER-TBI and TRACK-TBI cohorts
Abstract
Background: Post-traumatic stress disorder (PTSD) and depression are common after mild traumatic brain injury (mTBI), but their biological drivers are uncertain. We therefore explored whether polygenic risk scores (PRS) derived for PTSD and major depressive disorder (MDD) are associated with the development of cognate TBI-related phenotypes.
Methods: Meta-analyses were conducted using data from two multicenter, prospective observational cohort studies of patients with mTBI: the CENTER-TBI study (ClinicalTrials.gov ID NCT02210221) in Europe (December 2014-December 2017) and the TRACK-TBI study in the US (March 2014-July 2018). In both cohorts, the most common causes of injury were road traffic accidents and falls. Primary outcomes, specifically probable PTSD and depression, were defined at 6 months post-injury using scores ≥33 on the PTSD Checklist-5 and ≥15 on the Patient Health Questionnaire-9, respectively. We calculated PTSD-PRS and MDD-PRS for patients aged ≥17 years who had a Glasgow Coma Scale score of 13-15 upon hospital arrival and assessed their association with PTSD and depression following TBI. We also evaluated the transferability of the findings in a cohort of African Americans.
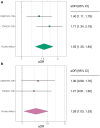
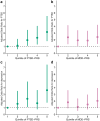
Findings: Overall, 11.8% (219/1869) and 6.7% (124/1869) patients were classified as having probable PTSD and depression, respectively. The PTSD-PRS was significantly associated with higher adjusted odds of PTSD in both cohorts, with a pooled odds ratio (OR) of 1.55 [95% confidence interval (CI) 1.30-1.84, p < 0.001, I 2 = 20.8%]. Although the MDD-PRS increased the risk of depression after TBI, it did not reach significance in the individual cohorts. However, in a combined analysis, the risk was significantly elevated with a pooled OR of 1.26 [95% CI 1.03-1.53, p = 0.02, I 2 = 0%]. The addition of PRSs improved the proportion of outcome variance explained in the two study cohorts from 19.5% and 30.3% to 21.6% and 34.0% for PTSD; and from 11.0% and 22.5% to 12.8% and 22.6% for depression. Patients in the highest cognate PRS quintile had increased odds of 3.16 [95% CI 1.80-5.55] and 2.03 [95% CI 1.04-3.94] of developing PTSD or depression compared to the lowest quintile, respectively.
Interpretation: Associations of PRSs with PTSD and depression following TBI are not disorder-specific. However, the overlap between MDD-PRS and depression following TBI is less robust compared to PTSD-PRS and PTSD. PRSs could improve risk prediction, and permit enrichment for interventional trials.
Funding: This study was supported by funding by an FP7 grant from the European Union, Hannelore Kohl Stiftung, Integra LifeSciences Corporation, NeuroTrauma Sciences, US National Institutes of Health, US Department of Defense, National Football League Advisory Board, US Department of Energy, and One Mind.
Keywords: Depression; Mental health; Polygenic risk score; Post-traumatic stress disorder; Traumatic brain injury.
© 2024 The Authors.
Conflict of interest statement
LW reports receiving consultancy fees from NeuroTrauma Sciences and Spaulding-Harvard TBI Model System outside the submitted work. JR declares payment for expert testimony from the National Football League. GTM has received funding from NeuroTrauma Sciences and One Mind. AIRM serves as an advisory board member for PressuraNeuro and declares consulting fees from Novartis and NeuroTrauma Sciences. MBS serves as a data and safety monitoring board member for the University of Nebraska and the University of Boston, declares royalties with UpToDate, stock options in Oxeia Biopharmaceuticals, and consulting fees from Acadia Pharmaceuticals, Aptinyx, atai Life Sciences, BigHealth, Biogen, Bionomics, BioXcel Therapeutics, Boehringer Ingelheim, Clexio, Delix Therapeutics, Eisai, EmpowerPharm, Engrail Therapeutics, Janssen, Jazz Pharmaceuticals, NeuroTrauma Sciences, PureTech Health, Sage Therapeutics, Sumitomo Pharma, and Roche/Genentech. DKM declares research collaborations or consultancy/lecture fees with regard to the following organisations: NeuroTrauma Sciences, Lantmannen AB, GlaxoSmithKline Ltd., PresSura Neuro, CSL Behring, Invex Ltd., and Integra Neurosciences Ltd. The remaining authors declare no competing interests.
Figures