Dual single‑nucleotide polymorphism biomarker combination for opioid selection to treat cancer pain
- PMID: 39720462
- PMCID: PMC11667419
- DOI: 10.3892/mco.2024.2809
Dual single‑nucleotide polymorphism biomarker combination for opioid selection to treat cancer pain
Abstract
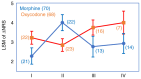
We have been exploring biomarkers that could help physicians select the appropriate opioid for individualized treatment of cancer pain. Recently, we identified a single nucleotide polymorphism (SNP) of CCL11 (rs17809012) as one such biomarker that was significantly associated with the analgesic effect of morphine. The current study measured the plasma concentrations of chemokines/cytokines in pre-treatment plasma samples of a total of 138 patients who were randomized to receive morphine (n=70) or oxycodone (n=68). Based on the results, one cytokine, IL-16, was identified whose plasma concentrations showed a clear bias between patients with cancer pain who responded well and responded poorly to oxycodone. A genotypic analysis also identified a SNP of the IL-16 gene (rs4778889) as being significantly associated with the analgesic effect of oxycodone. Whether both of the SNPs identified as being significant (CCL11 rs17809012 and IL-16 rs4778889) could be used in combination to accurately predict which opioid might be the most suitable to provide pain relief in patients with cancer was assessed. Morphine tended to provide superior analgesic effect over oxycodone in patients with the IL-16 rs4778889 TT genotype and the CCL11 rs17809012 AG/GG genotype (n=45), while a trend towards a greater analgesic effect of oxycodone was observed in patients with other genotype combinations of these SNPs (n=93) (P=0.0012 for the interaction), suggesting that the IL-16 rs4778889 and CCL11 rs17809012 SNPs could serve as a potential dual-biomarker combination for personalized analgesic therapy in patients with cancer pain.
Keywords: CCL11; IL-16; cancer pain; genotype; morphine; oxycodone; plasma concentration; single nucleotide polymorphism.
Copyright: © 2024 Fujita et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Crews KR, Monte AA, Huddart R, Caudle KE, Kharasch ED, Gaedigk A, Dunnenberger HM, Leeder JS, Callaghan JT, Samer CF, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin Pharmacol Ther. 2021;110:888–896. doi: 10.1002/cpt.2149. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
