The chicken cecal microbiome alters bile acids and riboflavin metabolism that correlate with intramuscular fat content
- PMID: 39720478
- PMCID: PMC11667789
- DOI: 10.3389/fmicb.2024.1494139
The chicken cecal microbiome alters bile acids and riboflavin metabolism that correlate with intramuscular fat content
Abstract
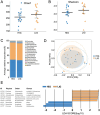
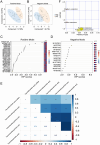
Intramuscular fat (IMF) is a key indicator of chicken meat quality and emerging studies have indicated that the gut microbiome plays a key role in animal fat deposition. However, the potential metabolic mechanism of gut microbiota affecting chicken IMF is still unclear. Fifty-one broiler chickens were collected to identify key cecal bacteria and serum metabolites related to chicken IMF and to explore possible metabolic mechanisms. The results showed that the IMF range of breast muscle of Guizhou local chicken was 1.65 to 4.59%. The complexity and stability of ecological network of cecal microbiota in low-IMF chickens were higher than those in high-IMF chickens. Cecal bacteria positively related to IMF were Alistipes, Synergistes and Subdoligranulum, and negatively related to IMF were Eubacterium_brachy_group, unclassified_f_Lachnospiraceae, unclassified_f_Coriobacteriaceae, GCA-900066575, Faecalicoccus, and so on. Bile acids, phosphatidylethanolamine (Pe) 32:1 and other metabolites were enriched in sera of high-IMF chickens versus low-IMF chickens while riboflavin was enriched in sera of low-IMF chickens. Correlation analysis indicated that specific bacteria including Alistipes promote deposition of IMF in chickens via bile acids while the Eubacterium_brachy group, and Coriobacteriaceae promoted formation of riboflavin, glufosinate, C10-dats (tentative), and cilastatin and were not conducive to the IMF deposition.
Keywords: cecal microbiota; chickens; integrative omics; intramuscular fat; metabolomics.
Copyright © 2024 Long, Zhang, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Cai Y., Weng K., Guo Y., Peng J., Zhu Z. J. (2015). An integrated targeted metabolomic platform for high-throughput metabolite profiling and automated data processing. Metabolomics 11, 1575–1586. doi: 10.1007/s11306-015-0809-4 - DOI
LinkOut - more resources
Full Text Sources

