Antithrombotic Therapy in Chronic Total Occlusion Interventions
- PMID: 39720495
- PMCID: PMC11664754
- DOI: 10.15420/usc.2020.37
Antithrombotic Therapy in Chronic Total Occlusion Interventions
Abstract
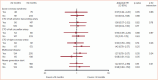
Chronic total occlusion (CTO) recanalization is among the most complex subsets of coronary interventions. Hence, optimum peri- and postprocedural anticoagulation and antiplatelet therapy is key for the achievement of successful revascularization and reduction of major adverse cardiovascular outcomes in patients undergoing CTO percutaneous coronary intervention (PCI). Unfractionated heparin is still considered the gold standard anticoagulant because its action can be reversed by protamine administration, with bivalirudin being reserved mainly for patients with heparin-induced thrombocytopenia. However, small studies comparing unfractionated heparin with bivalirudin in CTO interventions have shown similar outcomes. Glycoprotein IIb/IIIa inhibitors should, in general, be avoided. Aspirin in combination with clopidogrel for 6-12 months is the standard post CTO PCI dual antiplatelet regimen. For the most complex cases, clopidogrel can be substituted by a more potent P2Y12 inhibitor, namely ticagrelor or prasugrel.
Keywords: Chronic total occlusion; IIb/IIIa inhibitors; anticoagulation; antiplatelets; aspirin; bivalirudin; clopidogrel; percutaneous coronary intervention; prasugrel; ticagrelor; unfractionated heparin.
Copyright © The Author(s), 2021. Published by Radcliffe Group Ltd.
Conflict of interest statement
Disclosure: DA has received lecturing honoraria/advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim, Pfizer, Medtronic, Biotronik, and Chiesi Hellas; and is Guest Editor of the antithrombotics in high-risk PCI special collection for US Cardiology Review; this did not influence peer review. All other authors have no conflicts of interest to declare.
Figures
References
-
- Sapontis J, Salisbury AC, Yeh RW et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523–34. doi: 10.1016/j.jcin.2017.05.065. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous