Hyperaminoacidemia from interrupted glucagon signaling increases pancreatic acinar cell proliferation and size via mTORC1 and YAP pathways
- PMID: 39720531
- PMCID: PMC11667045
- DOI: 10.1016/j.isci.2024.111447
Hyperaminoacidemia from interrupted glucagon signaling increases pancreatic acinar cell proliferation and size via mTORC1 and YAP pathways
Abstract
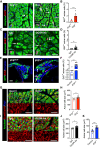
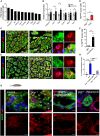
Increased blood amino acid levels (hyperaminoacidemia) stimulate pancreas expansion by unclear mechanisms. Here, by genetic and pharmacological disruption of glucagon receptor (GCGR) in mice and zebrafish, we found that the ensuing hyperaminoacidemia promotes pancreatic acinar cell proliferation and cell hypertrophy, which can be mitigated by a low protein diet in mice. In addition to mammalian target of rapamycin complex 1 (mTORC1) signaling, acinar cell proliferation required slc38a5, the most highly expressed amino acid transporter gene in both species. Transcriptomics data revealed the activation signature of yes-associated protein (YAP) in acinar cells of mice with hyperaminoacidemia, consistent with the observed increase in YAP-expressing acinar cells. Yap1 activation also occurred in acinar cells in gcgr-/- zebrafish, which was reversed by rapamycin. Knocking down yap1 in gcgr-/- zebrafish decreased mTORC1 activity and acinar cell proliferation and hypertrophy. Thus, the study discovered a previously unrecognized role of the YAP/Taz pathway in hyperaminoacidemia-induced acinar cell hypertrophy and hyperplasia.
Keywords: Biomolecules; Cell biology; Model organism; Molecular biology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Hyperglucagonaemia in diabetes: altered amino acid metabolism triggers mTORC1 activation, which drives glucagon production.Diabetologia. 2023 Oct;66(10):1925-1942. doi: 10.1007/s00125-023-05967-8. Epub 2023 Jul 22. Diabetologia. 2023. PMID: 37480416
-
Exploring the role of YAP1 and TAZ in pancreatic acinar cells and the therapeutic potential of VT-104 in pancreatic inflammation.J Pancreatol. 2025 Mar;8(1):32-40. doi: 10.1097/JP9.0000000000000170. Epub 2024 Jan 17. J Pancreatol. 2025. PMID: 40123617 Free PMC article.
-
Hippo pathway-mediated YAP1/TAZ inhibition is essential for proper pancreatic endocrine specification and differentiation.Elife. 2024 Jul 25;13:e84532. doi: 10.7554/eLife.84532. Elife. 2024. PMID: 39051998 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Rapamycin and rapalogs for tuberous sclerosis complex.Cochrane Database Syst Rev. 2016 Jul 13;7(7):CD011272. doi: 10.1002/14651858.CD011272.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Jul 11;7:CD011272. doi: 10.1002/14651858.CD011272.pub3. PMID: 27409709 Free PMC article. Updated.
References
-
- Wollny D., Zhao S., Everlien I., Lun X., Brunken J., Brüne D., Ziebell F., Tabansky I., Weichert W., Marciniak-Czochra A., Martin-Villalba A. Single-Cell Analysis Uncovers Clonal Acinar Cell Heterogeneity in the Adult Pancreas. Dev. Cell. 2016;39:289–301. doi: 10.1016/j.devcel.2016.10.002. - DOI - PubMed
-
- Tosti L., Hang Y., Debnath O., Tiesmeyer S., Trefzer T., Steiger K., Ten F.W., Lukassen S., Ballke S., Kühl A.A., et al. Single-Nucleus and In Situ RNA-Sequencing Reveal Cell Topographies in the Human Pancreas. Gastroenterology. 2021;160:1330–1344.e11. doi: 10.1053/j.gastro.2020.11.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

