Accumulation of alpha-synuclein pathology in the liver exhibits post-translational modifications associated with Parkinson's disease
- PMID: 39720536
- PMCID: PMC11667178
- DOI: 10.1016/j.isci.2024.111448
Accumulation of alpha-synuclein pathology in the liver exhibits post-translational modifications associated with Parkinson's disease
Abstract
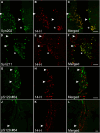
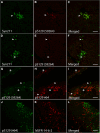
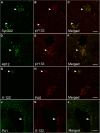
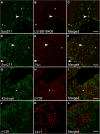
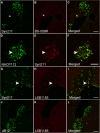
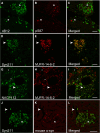
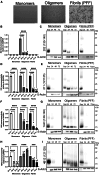
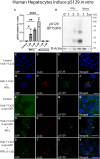
Accumulating evidence demonstrates that alpha-synuclein (α-syn) pathology associated with Parkinson's disease (PD) is not limited to the brain, as it also appears in a select number of peripheral tissues including the liver. In this study, we identified a number of PD-associated α-syn post-translational modifications in the livers of (Thy-1)-h[A30P] mice, a mouse model of familial PD expressing human α-syn harboring the A30P mutation driven by a neuron-specific promoter. Ex vivo, we also demonstrate that human hepatocytes induce post-translational modifications following α-syn fibrillar (PFF) treatment. Moreover, such cells also degrade PFFs over time, whereas oligomeric assemblies are more resistant to degradation, but this process can be enhanced by autophagy stimulators. Collectively, our findings suggest that pathological α-syn is transported to the liver in a modified state or is modified upon arrival, which facilitates its clearance and detoxification, pointing to a role for the liver in the degradation of PD-associated pathology.
Keywords: Biological sciences; Molecular neuroscience; Neuroscience.
© 2024 The Author(s).
Conflict of interest statement
MI is a paid consultant to BioArctic AB.
Figures


References
-
- Braak H., Rüb U., Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson's disease. J. Neurol. Sci. 2006;248:255–258. - PubMed
-
- Gasser T. Genetics of Parkinson's disease. Curr. Opin. Neurol. 2005;18:363–369. - PubMed
-
- Petrucci S., Ginevrino M., Valente E.M. Phenotypic spectrum of alpha-synuclein mutations: New insights from patients and cellular models. Parkinsonism Relat. Disorders. 2016;22:S16–S20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

