Immune regulatory adjuvant approach to mitigate subcutaneous immunogenicity of monoclonal antibodies
- PMID: 39720710
- PMCID: PMC11666448
- DOI: 10.3389/fimmu.2024.1496169
Immune regulatory adjuvant approach to mitigate subcutaneous immunogenicity of monoclonal antibodies
Abstract
Introduction: Immunogenicity continues to be a challenge for development and clinical utility of monoclonal antibodies, and there are gaps in our current ability to prevent anti-drug antibody development in a safe and antigen-specific manner.
Methods: To mitigate immunogenicity of monoclonal antibodies administered subcutaneously, O-phospho-L-serine (OPLS)-the head group of the tolerance-inducing phospholipid, phosphatidylserine-was investigated as an immunoregulatory adjuvant.
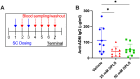
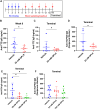
Results: Formulations of adalimumab, trastuzumab or rituximab with OPLS showed reduction in relative immunogenicity in mice compared to vehicle formulations, indicated by reduced anti-drug antibody development and significant reductions in CD138+ plasma cell differentiation in bone marrow. Titer development toward recombinant human hyaluronidase, a dispersion enhancer that was co-formulated with monoclonal antibodies, was similarly reduced. Subcutaneous administration of adalimumab with OPLS resulted in a two-fold increase in expression of type 1 regulatory (Tr1) T cell subset in the spleen. This is consistent with in vitro studies where co-culturing of dendritic cells primed with ovalbumin in the presence and absence of OPLS and antigen specific T-cells induced expression of Tr1 phenotype on live CD4+ T cells.
Conclusion: This adjuvant does not impact immune competence of non-human primates and mice, and repeated administration of the adjuvant does not show renal or hepatic toxicity. Formulation of monoclonal antibodies with the immunoregulatory adjuvant, OPLS, was found to be safe and effective at mitigating immunogenicity.
Keywords: anti-drug antibodies; formulation; immune tolerance; immunogenicity; protein therapeutics; subcutaneous administration.
Copyright © 2024 Jarvi, Patel, Shetty, Nguyen, Grasperge, Mager, Straubinger and Balu-Iyer.
Conflict of interest statement
Author NN was employed by the company Truvai Biosciences, LLC. Author DM was employed by the company Enhanced Pharmacodynamics, LLC. Authors SB, DM, and RS have financial interest in Truvai Biosciences, LLC. SB and KS are inventors on a patent for use of OPLS as an immunomodifier. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest. The authors declare that this study received funding from Truvai Biosciences, LLC. The funder had the following involvement in the study: study design.
Figures






References
-
- Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. (2013) 53:192–201. doi: 10.1177/0091270012436560 - DOI - PubMed
-
- Banugaria SG, Prater SN, McGann JK, Feldman JD, Tannenbaum JA, Bailey C, et al. . Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med. (2013) 15:123–31. doi: 10.1038/gim.2012.110 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

