Prevalence of fungal colonization among patients with psoriasis in difficult-to-treat areas: impact of apremilast on mycotic burden and clinical outcomes
- PMID: 39720714
- PMCID: PMC11666449
- DOI: 10.3389/fimmu.2024.1508489
Prevalence of fungal colonization among patients with psoriasis in difficult-to-treat areas: impact of apremilast on mycotic burden and clinical outcomes
Abstract
Introduction: Fungi, including Candida, may be a trigger or exacerbate psoriasis, especially in difficult to treat (DTT) areas, through the activation of IL-17/23 axis.
Methods: In this study, seventy patients with DDT psoriasis were enrolled to evaluate Candida species and/or other opportunistic fungi colonization rate at baseline (T0) and the impact of apremilast on fungal load, clinical outcome, serum cytokine levels and biochemical serum profile of patients after 16, 24 and 52 weeks of treatment.
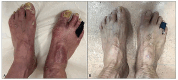
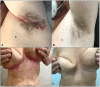
Results: In our population, 33 (47%) patients were colonized by Candida spp. at baseline. In 24 (34%) individuals Candida was detected in the oral cavity while in the remaining 9 (13%) individuals the fungus was isolated from stool samples. Twenty subjects were colonized by only the species C. albicans, whereas in the remaining 13 a combination of two or more species (C. albicans plus non-albicans strains) was found in the oral cavity. Moreover, 27 (39%) patients were affected by onychomycosis. At 52 weeks, apremilast treatment induced a full recovery from Candida colonization in 83% of patients colonized with a single species of Candida (C. albicans); while in those co-infected by two or more Candida spp. induced a significant reduction (colony counts >10 CFU/mL) in fungal load was observed in comparison to baseline. Among patients with onychomycosis, 78% (21/27) of them presented a complete clinical resolution of nail psoriasis and concomitant nail infections. Finally, improvements in clinical scores i.e., PASI, NAPSI, DLQI, itch VAS, PAIN VAS, scPGA and sPGA-G and biochemical serum profile, as well as a significant decrease in serum IL-17A, TGF-β 1 and IL-10 levels (from 8.51 to 4.16 pg/mL; from 66.10 to 48.70 ng/mL and from 20.05 to 14 pg/mL, respectively) were observed in all patients.
Conclusions: Fungi may play a role in the psoriasis pathogenesis. Apremilast has been shown to ameliorate psoriasis signs and symptoms and counteract fungal overgrowth, probably by dampening inflammation, triggered by the fungal infections themselves. Thus, apremilast may represent an effective therapeutic approach in the treatment of DTT psoriasis and modulate the fungal colonization.
Keywords: Candida species; IL-17; apremilast; cytokines; difficult-to-treat psoriasis areas; fungal infections.
Copyright © 2024 Campione, Cosio, Pistoia, Artosi, Shumack, Borselli, Rivieccio, Caputo, Favaro, Sorge, Pica, Bianchi and Gaziano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

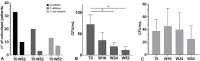



Similar articles
-
Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study.J Eur Acad Dermatol Venereol. 2018 Mar;32(3):397-402. doi: 10.1111/jdv.14738. Epub 2018 Jan 29. J Eur Acad Dermatol Venereol. 2018. PMID: 29220542 Free PMC article. Clinical Trial.
-
Apremilast mechanism of efficacy in systemic-naive patients with moderate plaque psoriasis: Pharmacodynamic results from the UNVEIL study.J Dermatol Sci. 2019 Dec;96(3):126-133. doi: 10.1016/j.jdermsci.2019.09.003. Epub 2019 Sep 10. J Dermatol Sci. 2019. PMID: 31787506 Clinical Trial.
-
Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: Results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2).J Am Acad Dermatol. 2016 Jan;74(1):134-42. doi: 10.1016/j.jaad.2015.09.001. J Am Acad Dermatol. 2016. PMID: 26549249 Clinical Trial.
-
Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis.J Am Acad Dermatol. 2016 Oct;75(4):740-746. doi: 10.1016/j.jaad.2016.05.040. Epub 2016 Jul 28. J Am Acad Dermatol. 2016. PMID: 27476973 Review.
-
Effective treatment of nail psoriasis with apremilast: report of two cases and review of the literature.Dermatol Online J. 2018 Sep 15;24(9):13030/qt27x34947. Dermatol Online J. 2018. PMID: 30677839 Review.
Cited by
-
PDE4 inhibitors in psoriasis therapy: current insights and future directions.Inflammopharmacology. 2025 Jun;33(6):2885-2906. doi: 10.1007/s10787-025-01778-y. Epub 2025 May 15. Inflammopharmacology. 2025. PMID: 40374992 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

