Hematopoietic stem cell a reservoir of innate immune memory
- PMID: 39720722
- PMCID: PMC11666435
- DOI: 10.3389/fimmu.2024.1491729
Hematopoietic stem cell a reservoir of innate immune memory
Abstract
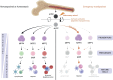
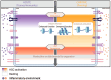
Hematopoietic stem cells (HSCs) are a rare, long-lived and multipotent population that give rise to majority of blood cells and some tissue-resident immune cells. There is growing evidence that inflammatory stimuli can trigger persistent reprogramming in HSCs that enhances or inhibits the cellular functions of these HSCs and their progeny in response to subsequent infections. This newly discovered property makes HSCs a reservoir for innate immune memory. The molecular mechanisms underlying innate immune memory in HSCs are similar to those observed in innate immune cells, although their full elucidation is still pending. In this review, we examine the current state of knowledge on how an inflammatory response leads to reprogramming of HSCs. Understanding the full spectrum of consequences of reshaping early hematopoiesis is critical for assessing the potential benefits and risks under physiological and pathological conditions.
Keywords: emergency hematopoiesis; epigenetic; hematopoietic stem and progenitor cells (HSPCs); inflammation; innate immune memory; metabolism; myelopoiesis.
Copyright © 2024 Ruffinatto, Groult, Iacono, Sarrazin and de Laval.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

