Understanding Cognitive Aging Through White Matter: A Fixel-Based Analysis
- PMID: 39720841
- PMCID: PMC11669003
- DOI: 10.1002/hbm.70121
Understanding Cognitive Aging Through White Matter: A Fixel-Based Analysis
Abstract
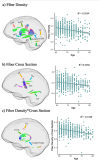
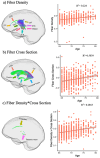
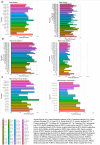
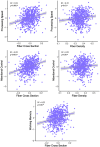
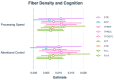
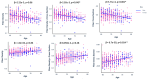
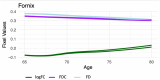
Diffusion-weighted imaging (DWI) has been frequently used to examine age-related deterioration of white matter microstructure and its relationship to cognitive decline. However, typical tensor-based analytical approaches are often difficult to interpret due to the challenge of decomposing and (mis)interpreting the impact of crossing fibers within a voxel. We hypothesized that a novel analytical approach capable of resolving fiber-specific changes within each voxel (i.e., fixel-based analysis [FBA])-would show greater sensitivity relative to the traditional tensor-based approach for assessing relationships between white matter microstructure, age, and cognitive performance. To test our hypothesis, we studied 636 cognitively normal adults aged 65-80 years (mean age = 69.8 years; 71% female) using diffusion-weighted MRI. We analyzed fixels (i.e., fiber-bundle elements) to test our hypotheses. A fixel provides insight into the structural integrity of individual fiber populations in each voxel in the presence of multiple crossing fiber pathways, allowing for potentially increased specificity over other diffusion measures. Linear regression was used to investigate associations between each of three fixel metrics (fiber density, cross-section, and density × cross-section) with age and cognitive performance. We then compared and contrasted the FBA results to a traditional tensor-based approach examining voxel-wise fractional anisotropy. In a whole-brain analysis, significant associations were found between fixel-based metrics and age after adjustments for sex, education, total brain volume, site, and race. We found that increasing age was associated with decreased fiber density and cross-section, namely in the fornix, striatal, and thalamic pathways. Further analysis revealed that lower fiber density and cross-section were associated with poorer performance in measuring processing speed and attentional control. In contrast, the tensor-based analysis failed to detect any white matter tracts significantly associated with age or cognition. Taken together, these results suggest that FBAs of DWI data may be more sensitive for detecting age-related white matter changes in an older adult population and can uncover potentially clinically important associations with cognitive performance.
Keywords: DWI; aging; cognitive decline; fixel‐based analysis; white matter.
© 2024 The Author(s). Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

