HBV-Induced Carcinogenesis: Mechanisms, Correlation With Viral Suppression, and Implications for Treatment
- PMID: 39720865
- PMCID: PMC11669079
- DOI: 10.1111/liv.16202
HBV-Induced Carcinogenesis: Mechanisms, Correlation With Viral Suppression, and Implications for Treatment
Abstract
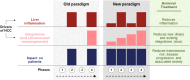
Background: Chronic hepatitis B virus (HBV) infection is a common but underdiagnosed and undertreated health condition and is the leading cause of hepatocellular carcinoma (HCC) worldwide. HBV (rated a Grade 1 carcinogen by the International Agency for Research on Cancer) drives the transformation of hepatocytes in multiple ways by inducing viral DNA integrations, genetic dysregulation, chromosomal translocations, chronic inflammation, and oncogenic pathways facilitated by some HBV proteins. Importantly, these mechanisms are active throughout all phases of HBV infection. Nevertheless, most clinical guidelines for antiviral therapy recommend treatment based on a complex combination of HBV DNA levels, transaminasemia, liver histology, and demographic factors, rather than prompt treatment for all people with infection.
Aims: To determine if current frameworks for antiviral treatment address the impacts of chronic HBV infection particularly preventing cancer development.
Materials and methods: We reviewed the recent data demonstrating pro-oncogenic factors acting throughout a chronic HBV infection can be inhibited by antiviral therapy.
Results: We extensively reviewed Hepatitis B virology data and correlating clinical outcome data. From thi, we suggest that new findings support simplifying and expanding treatment initiation to reduce the incidence ofnew infections, progressive liver disease, and risk of hepatocellular carcinoma. We also consider lessons learned from other blood-borne pathogens, including the benefits of antiviral treatment in preventing transmission, reducing stigma, and reframing treatment as cancer prevention.
Conclusion: Incorporating these practice changes into treatment is likely to reduce the overall burden of chronic HBV infections and HCC. Through this, we may better achieve the World Health Organization's goal of eliminating viral hepatitis as a public health threat and minimise its impact on people's lives.
Keywords: HBV infection; antiviral therapy; chronic hepatitis B virus; hepatocellular carcinoma.
© 2024 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
T.T. reports receiving grant funding from Excision Biosciences and Gilead Sciences Inc.; is president of the Australian Centre for Hepatitis Virology; is founder/director of
Figures

References
-
- World Health Organization , Hepatitis B (Geneva, Switzerland: World Health Organization, 2020).
-
- Le M. H., Yeo Y. H., Cheung R., Henry L., Lok A. S., and Nguyen M. H., “Chronic Hepatitis B Prevalence Among Foreign‐Born and U.S.‐Born Adults in the United States, 1999–2016,” Hepatology 71, no. 2 (2020): 431–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

