Integrated Analysis of Vonoprazan Safety for Symptomatic Gastro-Oesophageal Reflux Disease or Erosive Oesophagitis
- PMID: 39720884
- PMCID: PMC11825931
- DOI: 10.1111/apt.18458
Integrated Analysis of Vonoprazan Safety for Symptomatic Gastro-Oesophageal Reflux Disease or Erosive Oesophagitis
Abstract
Background: Patients with erosive oesophagitis, and those with persistent symptomatic non-erosive gastro-oesophageal reflux disease, require long-term maintenance treatment with acid-suppressing agents.
Aim: To evaluate the safety of vonoprazan, a potassium-competitive acid blocker, in an integrated analysis of data from clinical trials in adults.
Methods: We included 14 clinical trials of vonoprazan conducted in multiple countries. Mean duration of exposure in person-years to vonoprazan (n = 5318) was 2068, to comparators lansoprazole (n = 1925) or esomeprazole (n = 86) was 751, and to placebo (n = 779) was 59. We report adverse events, serum gastrin, and liver enzyme levels as the main outcomes. Post-marketing safety data from December 26, 2014 (date of commercialisation in Japan) to December 25, 2023, are also provided.
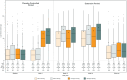
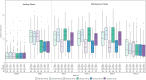
Results: Nasopharyngitis was the only adverse event reported by at least 5.0% of patients (6.94% vonoprazan, 5.07% proton pump inhibitor (PPI), 4.49% placebo). Incidence rates per 100 person-years for serious adverse events were 10.39 for vonoprazan, 10.65 for PPIs, and 1.69 for placebo. One patient each on vonoprazan and lansoprazole was diagnosed with gastric cancer. Mean serum gastrin levels were higher on vonoprazan than lansoprazole but normalised by 4 weeks after discontinuation. Elevated liver enzyme levels were infrequent and of low magnitude with no differences between vonoprazan and PPIs. There were four deaths; none was considered related to study drug.
Conclusions: Vonoprazan was well tolerated. Its safety profile from both clinical trial and post-marketing data were consistent and comparable to that of its PPI comparators with respect to treatment-emergent adverse events.
Keywords: erosive oesophagitis; gastro‐oesophageal reflux disease; potassium‐competitive acid blocker; vonoprazan.
© 2024 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
C.W.H. is a consultant for Phathom Pharmaceuticals and Sebela; P.K. is a consultant for Phathom Pharmaceuticals and Sebela; K.R.D. is a consultant for Phathom Pharmaceuticals; D.C.M. has no conflicts to report; D.T. is an employee of Takeda Pharmaceuticals International Co.; N.S., B.H., and Y.M.C. are employees of Phathom Pharmaceuticals; S.J.S. is a consultant for Phathom Pharmaceuticals, Takeda Pharmaceuticals, and Castle Biosciences.
Figures
References
-
- Richter J., Castell D., and Katzka D., The Esophagus, 6th ed. (Hoboken, New Jersey, USA: John Wiley & Sons Ltd, 2021).
-
- Vaezi M. F., Yang Y. X., and Howden C. W., “Complications of Proton Pump Inhibitor Therapy,” Gastroenterology 153, no. 1 (2017): 35–48. - PubMed
-
- Moayyedi P., Eikelboom J. W., Bosch J., et al., “Safety of Proton Pump Inhibitors Based on a Large, Multi‐Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin,” Gastroenterology 157, no. 3 (2019): 682–691.e2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
