Copper(II) Oxide Spindle-like Nanomotors Decorated with Calcium Peroxide Nanoshell as a New Nanozyme with Photothermal and Chemodynamic Functions Providing ROS Self-Amplification, Glutathione Depletion, and Cu(I)/Cu(II) Recycling
- PMID: 39720911
- PMCID: PMC11783533
- DOI: 10.1021/acsami.4c17852
Copper(II) Oxide Spindle-like Nanomotors Decorated with Calcium Peroxide Nanoshell as a New Nanozyme with Photothermal and Chemodynamic Functions Providing ROS Self-Amplification, Glutathione Depletion, and Cu(I)/Cu(II) Recycling
Abstract
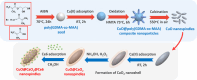
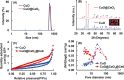
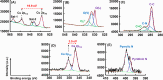
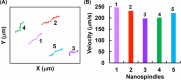
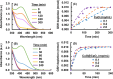
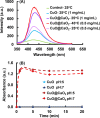
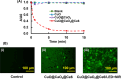
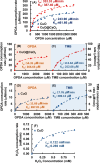
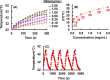
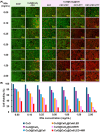
Uniform, mesoporous copper(II) oxide nanospindles (CuO NSs) were synthesized via a method based on templated hydrothermal oxidation of copper in the presence of monodisperse poly(glycerol dimethacrylate-co-methacrylic acid) nanoparticles (poly(GDMA-co-MAA) NPs). Subsequent decoration of CuO NSs with a CaO2 nanoshell (CuO@CaO2 NSs) yielded a nanozyme capable of Cu(I)/Cu(II) redox cycling. Activation of the Cu(I)/Cu(II) cycle by exogenously generated H2O2 from the CaO2 nanoshell significantly enhanced glutathione (GSH) depletion. CuO@CaO2 NSs exhibited a 2-fold higher GSH depletion rate compared to pristine CuO NSs. The generation of oxygen due to the catalase (CAT)-like decomposition of H2O2 by CuO@CaO2 NSs resulted in a self-propelled diffusion behavior, characteristic of a H2O2 fueled nanomotor. These nanostructures exhibited both peroxidase (POD)-like and CAT-like activities and were capable of self-production of H2O2 in aqueous media via a chemical reaction between the CaO2 nanoshell and water. Usage of the self-supplied H2O2 by the POD-like activity of CuO@CaO2 NSs amplified the generation of toxic hydroxyl (•OH) radicals, enhancing the chemodynamic effect within the tumor microenvironment (TME). The CAT-like activity provided a source of self-supplied O2 via decomposition of H2O2 to alleviate hypoxic conditions in the TME. Under near-infrared laser irradiation, CuO@CaO2 NSs exhibited photothermal conversion properties, with a temperature elevation of 25 °C. The combined GSH depletion and H2O2 generation led to a more effective production of •OH radicals in the cell culture medium. The chemodynamic function was further enhanced by an elevated temperature. To assess the therapeutic potential, CuO@CaO2 NSs loaded with the photosensitizer, chlorine e6 (Ce6), were evaluated against T98G glioblastoma cells. The synergistic combination of photodynamic, photohermal, and chemodynamic modalities using CuO@CaO2@Ce6 NSs resulted in cell death higher than 90% under in vitro conditions.
Keywords: Fenton-like reaction; chemodynamic therapy; glutathione depletion; nanozyme; peroxidase-like activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
GSH-Depleting and H2O2 Self-Supplying Calcium Peroxide-Based Nanoplatforms for Efficient Bacterial Eradication via Photothermal-Enhanced Chemodynamic Therapy.ACS Appl Mater Interfaces. 2024 Dec 18;16(50):69055-69070. doi: 10.1021/acsami.4c17388. Epub 2024 Dec 6. ACS Appl Mater Interfaces. 2024. PMID: 39641780
-
A ROS storm generating nanocomposite for enhanced chemodynamic therapy through H2O2 self-supply, GSH depletion and calcium overload.Nanoscale. 2024 May 2;16(17):8479-8494. doi: 10.1039/d3nr06422k. Nanoscale. 2024. PMID: 38590261
-
Engineering H2O2 Self-Supplying Platform for Xdynamic Therapies via Ru-Cu Peroxide Nanocarrier: Tumor Microenvironment-Mediated Synergistic Therapy.ACS Appl Mater Interfaces. 2024 May 15;16(19):24172-24190. doi: 10.1021/acsami.3c18888. Epub 2024 Apr 30. ACS Appl Mater Interfaces. 2024. PMID: 38688027 Free PMC article.
-
H2O2/O2 Self-Supplied Nanoplateform for amplifying oxidative stress to Accelerate Photodynamic/Chemodynamic therapy Cycles.J Colloid Interface Sci. 2025 Jul 15;690:137291. doi: 10.1016/j.jcis.2025.137291. Epub 2025 Mar 10. J Colloid Interface Sci. 2025. PMID: 40086336
-
Open-Source and Reduced-Expenditure Nanosystem with ROS Self-Amplification and Glutathione Depletion for Simultaneous Augmented Chemodynamic/Photodynamic Therapy.ACS Appl Mater Interfaces. 2022 May 11;14(18):20682-20692. doi: 10.1021/acsami.2c01782. Epub 2022 May 2. ACS Appl Mater Interfaces. 2022. PMID: 35500204
Cited by
-
Nanoparticles for Glioblastoma Treatment.Pharmaceutics. 2025 May 23;17(6):688. doi: 10.3390/pharmaceutics17060688. Pharmaceutics. 2025. PMID: 40574001 Free PMC article. Review.
References
-
- You K.; Wang Q.; Osman M. S.; Kim D.; Li Q.; Feng C.; Wang L.; Yang K. Advanced strategies for combinational immunotherapy of cancer based on polymeric nanomedicines. BMEMat 2024, 2, e1206710.1002/bmm2.12067. - DOI
-
- He S.-B.; Balasubramanian P.; Hu A.-L.; Zheng X.-Q.; Lin M.-T.; Xiao M.-X.; Peng H.-P.; Deng H.-H.; Chen W. One-pot cascade catalysis at neutral pH driven by CuO tandem nanozyme for ascorbic acid and alkaline phosphatase detection. Sens. Actuators, B 2020, 321, 128511.10.1016/j.snb.2020.128511. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

