PWP1 transcriptionally regulates p53, modulating apoptosis and cell cycle to promote gastric cancer progression
- PMID: 39720977
- PMCID: PMC11947051
- DOI: 10.1007/s10495-024-02049-x
PWP1 transcriptionally regulates p53, modulating apoptosis and cell cycle to promote gastric cancer progression
Abstract
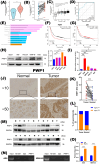
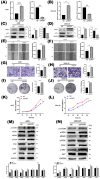
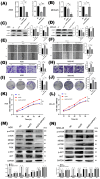
Gastric cancer remains a leading cause of cancer-related mortality worldwide. The prognosis often depends on early detection and understanding the molecular mechanisms involved in its progression. Periodic tryptophan protein 1 (PWP1) has emerged as a novel diagnostic marker, potentially linked to gastric cancer progression. This study aims to elucidate the impact of PWP1 on gastric cancer development, focusing on apoptosis, cell cycle regulation, and the role of p53. This study utilized gastric cancer cell lines to investigate the expression and functional role of Pwp1. Quantitative PCR and Western blot analyses were conducted to measure PWP1 expression levels. Apoptosis was assessed by using flow cytometry and TUNEL assays, and cell cycle analysis was performed to evaluate the impact of PWP1 modulation. Additionally, animal experiments were conducted using mouse models injected with gastric cancer cells, with PWP1 knockdown or overexpression, to observe tumor growth and progression. Statistical significance was evaluated using t-tests and ANOVA where appropriate. Elevated PWP1 expression was observed in gastric cancer tissues compared to normal tissues. PWP1's knockdown resulted in increased apoptosis and cell cycle arrest at the G1 phase, suggesting its role in promoting invasion and proliferation. Furthermore, animal experiments demonstrated reduced tumor growth in mice with PWP1 knockdown. PWP1 was found to transcriptionally regulate p53, affecting its expression and thereby influencing apoptosis and cell cycle pathways in gastric cancer. Our study identifies PWP1 as a novel oncogene frequently overexpressed in gastric cancer (GC). Through transcriptional regulation of p53, PWP1 enhances cell growth by influencing apoptosis and inducing G1 phase cell cycle arrest. These findings underscore PWP1 as a promising therapeutic target for treating GC, suggesting its potential for future clinical applications.
Keywords: Apoptosis; Cell cycle; Gastric cancer; PWP1; p53.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors declare no conflict of interest. Ethics approval and consent to participate: Approval of the research protocol by the Ethics Committee of North Jiangsu People’s Hospital with the ethics number 2020KY-137. All institutional and national guidelines for the care and use of laboratory animals were followed. And approved by Yangzhou University (NO.202307001). Consent for publication: Not applicable.
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 - PubMed
-
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F (2020) Gastric cancer. Lancet 396(10251):635–648 - PubMed
-
- Reed JC (1999) Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol 11(1):68–75 - PubMed
-
- Sherr CJ (1996) Cancer cell cycles. Science 274(5293):1672–1677 - PubMed
-
- Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8(4):275–283 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

