Lactylation of Histone H3k18 and Egr1 Promotes Endothelial Glycocalyx Degradation in Sepsis-Induced Acute Lung Injury
- PMID: 39721014
- PMCID: PMC11831459
- DOI: 10.1002/advs.202407064
Lactylation of Histone H3k18 and Egr1 Promotes Endothelial Glycocalyx Degradation in Sepsis-Induced Acute Lung Injury
Abstract
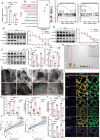
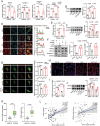
Circulating lactate is a critical biomarker for sepsis-induced acute lung injury (S-ALI) and is strongly associated with poor prognosis. However, whether elevated lactate directly promotes S-ALI and the specific mechanism involved remain unclear. Here, this work shows that lactate causes pulmonary endothelial glycocalyx degradation and worsens ALI during sepsis. Mechanistically, lactate increases the lactylation of K18 of histone H3, which is enriched at the promoter of EGR1 and promotes its transcription, leading to upregulation of heparanase in pulmonary microvascular endothelial cells. In addition, multiple lactylation sites are identified in EGR1, and lactylation is confirmed to occur mainly at K364. K364 lactylation of EGR1 facilitates its interaction with importin-α, in turn promoting its nuclear localization. Importantly, this work identifies KAT2B as a novel lactyltransferase whose GNAT domain directly mediates the lactylation of EGR1 during S-ALI. In vivo, suppression of lactate production or genetic knockout of EGR1 mitigated glycocalyx degradation and ALI and improved survival outcomes in mice with polymicrobial sepsis. Therefore, this study reveals that the crosstalk between metabolic reprogramming in endothelial cells and epigenetic modifications plays a critical role in the pathological processes of S-ALI.
Keywords: EGR1; KAT2B; glycocalyx degradation; lactylation; sepsis‐induced acute lung injury.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C. M., French C., Machado F. R., Mcintyre L., Ostermann M., Prescott H. C., Schorr C., Simpson S., Wiersinga W. J., Alshamsi F., Angus D. C., Arabi Y., Azevedo L., Beale R., Beilman G., Belley‐Cote E., Burry L., Cecconi M., Centofanti J., Coz Yataco A., De Waele J., Dellinger R. P., Doi K., Du B., Estenssoro E., Ferrer R., et al., Intensive Care Med. 2021, 47, 1181. - PMC - PubMed
-
- Xie J., Wang H., Kang Y., Zhou L., Liu Z., Qin B., Ma X., Cao X., Chen D., Lu W., Yao C., Yu K., Yao X., Shang H., Qiu H., Yang Y., Crit. Care Med. 2020, 48, e209. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
