Berberine Derivative B68 Promotes Tumor Immune Clearance by Dual-Targeting BMI1 for Senescence Induction and CSN5 for PD-L1 Degradation
- PMID: 39721027
- PMCID: PMC11831439
- DOI: 10.1002/advs.202413122
Berberine Derivative B68 Promotes Tumor Immune Clearance by Dual-Targeting BMI1 for Senescence Induction and CSN5 for PD-L1 Degradation
Abstract
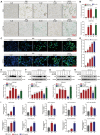
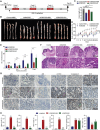
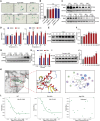
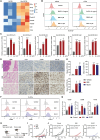
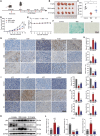
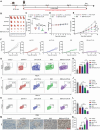
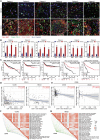
Promoting tumor cell senescence arrests the cell cycle of tumor cells and activates the immune system to eliminate these senescent cells, thereby suppressing tumor growth. Nevertheless, PD-L1 positive senescent tumor cells resist immune clearance and possess the ability to secret various cytokines and inflammatory factors that stimulate the growth of tumor cells. Consequently, drugs capable of both triggering senescence in tumor cells and concurrently diminishing the expression of PD-L1 to counteract immune evasion are urgently needed. Here, a berberine derivative B68 is developed, which specifically induces tumor cell senescence by targeting BMI1. B68 also involves the degradation of PD-L1 by targeting CSN5, thereby disrupting the immunosuppressive PD-1/PD-L1 interaction and enabling rapid clearance of senescent tumor cells. This approach simultaneously inhibits tumor progression and activates T cell immunity, as evidenced by the robust antitumor response following B68-induced immunization of senescent cancer cells. Moreover, the synergistic effect of B68 with anti-CTLA4 therapy further enhances antitumor immunity, and its ability to induce senescence in cancer cells triggers a strong protective response by dendritic and CD8+ T cells. These findings provide a scientific basis for developing a new tumor treatment strategy based on senescence induction and lay the foundation for further preclinical research.
Keywords: BMI1; CSN5; PD‐L1; colorectal cancer; tumor cell senescence.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 82374086/National Natural Science Foundation of China
- 82141203/National Natural Science Foundation of China
- 82104459/National Natural Science Foundation of China
- 22077131/National Natural Science Foundation of China
- 22277129/National Natural Science Foundation of China
- 2022YFC3502000/National Key Research and Development Program of China
- ZD2021CY001/Shanghai Municipal Science and Technology Major Project
- ZYYCXTDD-202004/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- 20YF1458700/Science and Technology Commission of Shanghai Municipality
- 2023YZZ02/Organizational Key Research and Development Program of Shanghai University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical
Research Materials
