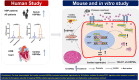
Cardiac secreted HSP90α exacerbates pressure overload myocardial hypertrophy and heart failure
- PMID: 39721497
- PMCID: PMC11732234
- DOI: 10.1016/j.redox.2024.103466
Cardiac secreted HSP90α exacerbates pressure overload myocardial hypertrophy and heart failure
Abstract
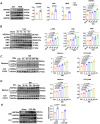
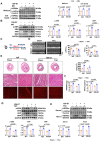
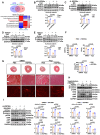
Sustained myocardial hypertrophy or left ventricular hypertrophy (LVH) triggered by pressure overload is strongly linked to adverse cardiovascular outcomes. Here, we investigated the clinical relationship between serum HSP90α (an isoform of HSP90) levels and LVH in patients with hypertension or aortic stenosis (AS) and explored underlying mechanisms in pressure overload mouse model. We built a pressure overload mouse model via transverse aortic constriction (TAC). Compared to controls, elevated serum HSP90α levels were observed in patients with hypertension or AS, and the levels positively correlated with LVH. Similarly, HSP90α levels increased in heart tissues from patients with obstructive hypertrophic cardiomyopathy (HCM), and in mice post-TAC. TAC induced the enhanced cardiac expression and secretion of HSP90α from cardiomyocytes and cardiac fibroblasts. Knockdown of HSP90α or blockade of extracellular HSP90α (eHSP90α) attenuated cardiac hypertrophy and dysfunction by inhibition of β-catenin/TCF7 signaling under pressure overload. Further analysis revealed that eHSP90α interacted with EC1-EC2 region of N-cadherin to activate β-catenin, enhancing the transcription of hypertrophic genes by TCF7, resulting in cardiac hypertrophy and dysfunction under pressure overload. These insights suggest the therapeutic potential of targeting HSP90α-initiated signaling pathway against cardiac hypertrophy and heart failure under pressure overload.
Keywords: Cardiac hypertrophy; HSP90; Heart failure; Pressure overload; β-catenin.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Felix-Redondo F.J., Fernandez-Berges D., Calderon A., et al. Prevalence of left-ventricular hypertrophy by multiple electrocardiographic criteria in general population: hermex study. J. Hypertens. 2012;30(7):1460–1467. - PubMed
-
- Cuspidi C., Sala C., Negri F., et al. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J. Hum. Hypertens. 2012;26(6):343–349. - PubMed
-
- Cioffi G., Faggiano P., Vizzardi E., et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97(4):301–307. - PubMed
-
- Tham Y.K., Bernardo B.C., Ooi J.Y., et al. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015;89(9):1401–1438. - PubMed
-
- Richter K., Buchner J. Hsp90: chaperoning signal transduction. J. Cell. Physiol. 2001;188(3):281–290. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

