Atherogenic Dyslipidemia Is Critically Related to Aortic Complicated Lesions in Cryptogenic Stroke
- PMID: 39721702
- PMCID: PMC12237763
- DOI: 10.5551/jat.65289
Atherogenic Dyslipidemia Is Critically Related to Aortic Complicated Lesions in Cryptogenic Stroke
Abstract
Aims: Atherogenic dyslipidemia (AD) is regarded as a residual risk of cardiovascular diseases characterized by low high-density lipoprotein cholesterol (HDL-C) and high triglyceride (TG) levels and related to the intracranial stenosis of atheromatous thrombotic brain infarction (ATBI). Further, atherosclerosis is possibly related to another stroke subtype, including cryptogenic stroke (CS). In particular, an aortic complicated lesion (ACL) is a notable embolic source of CS, since recurrence of aortogenic brain embolism is not rare. This study aimed to clarify the underlying association between AD and CS.
Methods: CHALLENGE ESUS/CS (Mechanisms of Embolic Stroke Clarified by Transesophageal Echocardiography for ESUS/CS) had extensive data from CS patients who underwent transesophageal echocardiography (TEE). AD was defined as HDL-C ≤ 40 mg/dl and TG ≥ 150 mg/dl. Based on these criteria, patients were divided into an AD group and a non-AD group to compare the clinical features.
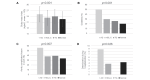
Results: Of 664 CS patients (446 men, 68.7±12.8 years), 68 (10.2%) met the criteria of AD (AD group), and 596 (89.8%) were in the non-AD group. On multiple logistic regression analysis, body mass index (unit OR 1.11, 95%CI 1.04-1.19, p=0.002), diabetes mellitus (OR 2.23, 95%CI 1.28-3.87, p=0.004), ACL in the arch (OR 1.89, 95%CI 1.09-3.31, p=0.025), and deterioration during hospitalization (OR 3.96, 95%CI 1.32-10.68, p=0.009) were independently associated with AD.
Conclusion: AD was not rare in the present CS population. Moreover, AD was crucially related to ACL in CS. Therefore, intensive and pleiotropic lipid-modifying therapy would be efficacious for further treatment of aortogenic brain embolism.
Keywords: Aortic complicated lesion; Atherogenic dyslipidemia; Cryptogenic stroke; Embolic stroke of undetermined source; Transesophageal echocardiography.
Conflict of interest statement
YU received lecture fees from OHARA Pharmaceutical Co., Ltd. and Daiichi Sankyo Co., Ltd. HT received lecture fees from Pfizer Japan Inc. and Daiichi Sankyo Co., Ltd. MK received honoraria from Daiichi-Sankyo Co., Ltd. and research support from Nippon Boehringer Ingelheim. YK received lecture fees from Daiichi Sankyo Co., Ltd., Medtronic CO. Ltd., Bayer Healthcare CO. Ltd. MI received lecture fees from Daiichi Sankyo Co., Ltd., Eisai Co. Ltd., and Bayer Pharmaceutical Co and a research grant from Shimadzu Corporation, Otsuka Pharmaceutical, and Panasonic Corporation. KH received lecture fees from Amgen Astellas BioPharma K.K., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd. YH received lecture fees from Bayer Pharmaceutical Co. and Nippon Boehringer Ingelheim, Co., Ltd. NH was an advisory member of Dai-Nippon Sumitomo Pharma Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., and Biogen Idec Japan Ltd., received lecture fees from Dai-Nippon Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical, Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kyowa Hakko-Kirin Co., Ltd., FP Pharmaceutical Corporation, Eisai Co., Ltd., Novartis Pharma K.K., and AbbVie, and received departmental endowments by commercial entities from Kyowa Hakko-Kirin Co., Ltd., Nippon Boehringer Ingelheim, Co., Ltd., AbbVie GK, FP Pharmaceutical Corporation, Otsuka Pharmaceutical, Co., Ltd., Dai-Nippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Nihon Medi-physics Co., Ltd., Asahi Kasei Medical Co., Ltd., Ono Pharmaceutical Co., Ltd., MiZ Co., Ltd., AbbVie GK, OHARA Pharmaceutical Co., Ltd., Nihon Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Boston Scientific Corporation, and Medtronic Inc. HT received lecture fees from Takeda Pharmaceutical CO., Ltd., Eisai CO., Ltd. NH received Stock holdings from PARKINSON Laboratories Co., Ltd, Stock option from NYSNOBIO GT NEUROLOGY, LLC, honoraria from Sumitomo Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., FP Corp., Eisai Co., Ltd., Nihon Medi-physics Co., Ltd., Novartis Pharma K.K., Biogen Idec Japan Ltd., AbbVie GK, Teijin Pharma Limited, Alexion Pharmaceuticals, Inc., Daiichi Sankyo Co., Ltd. and Teijin Pharma Limited, grants from Asahi Kasei Medical Co. Ltd., SNBL, Ltd, and funds for contract research from CellSource Co., Ltd., MJFF and MDS, scholarship grants from FP Corp. and Eisai Co., Ltd., and donations to the department, endowed research departments and joint collaborative research departments from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical, Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., SUNWELS Co., Ltd., Eisai Co., Ltd., Nihon Medi-physics Co., Ltd., Abbott Japan LLC , AbbVie GK, Medtronic, Inc., Boston Scientific Japan K.K., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., ZEBRA CO., LTD., KOWA Co., LTD, PARKINSON Laboratories Co., Ltd and OHARA Pharmaceutical Co., Ltd. Outside the submitted work. TU received lecture fees from Daiichi Sankyo Co., Ltd., Boehringer Ingelheim, Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical CO., Ltd.and research funds from Otsuka Pharmaceutical Co., Ltd. and AbbVie GK.
Figures


References
-
- Iso H, Jacobs DR, Jr., Wentworth D, Neaton JD, Cohen JD: Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med, 1989; 320: 904-910 - PubMed
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists C: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 - PubMed
-
- Amarenco P, Labreuche J: Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol, 2009; 8: 453-463 - PubMed
-
- Sirimarco G, Labreuche J, Bruckert E, Goldstein LB, Fox KM, Rothwell PM, Amarenco P, Perform, Investigators S, Committees: Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke, 2014; 45: 1429-1436 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

