Identification of Brain Cell Type-Specific Therapeutic Targets for Glioma From Genetics
- PMID: 39722126
- PMCID: PMC11669572
- DOI: 10.1111/cns.70185
Identification of Brain Cell Type-Specific Therapeutic Targets for Glioma From Genetics
Abstract
Background: Previous research has demonstrated correlations between the complex types and functions of brain cells and the etiology of glioma. However, the causal relationship between gene expression regulation in specific brain cell types and glioma risk, along with its therapeutic implications, remains underexplored.
Methods: Utilizing brain cell type-specific cis-expression quantitative trait loci (cis-eQTLs) and glioma genome-wide association study (GWAS) datasets in conjunction with Mendelian randomization (MR) and colocalization analyses, we conducted a systematic investigation to determine whether an association exists between the gene expression of specific brain cell types and the susceptibility to glioma, including its subtypes. Additionally, the potential pathogenicity was explored utilizing mediation and bioinformatics analyses. This exploration ultimately led to the identification of a series of brain cell-specific therapeutic targets.
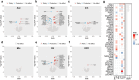
Results: A total of 110 statistically significant and robust associations were identified through MR analysis, with most genes exhibiting causal effects exclusively in specific brain cell types or glioma subtypes. Bayesian colocalization analysis validated 36 associations involving 26 genes as potential brain cell-specific therapeutic targets. Mediation analysis revealed genes indirectly influencing glioma risk via telomere length. Bioinformatics analysis highlighted the involvement of these genes in glioma pathogenesis pathways and supported their enrichment in specific brain cell types.
Conclusions: This study, employing an integrated approach, demonstrated the genetic susceptibility between brain cell-specific gene expression and the risk of glioma and its subtypes. Its findings offer novel insights into glioma etiology and underscore potential therapeutic targets specific to brain cell types.
Keywords: Bayesian colocalization; Mendelian randomization; brain cells; glioma; therapeutic target.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Gritsch S., Batchelor T. T., and Gonzalez Castro L. N., “Diagnostic, Therapeutic, and Prognostic Implications of the 2021 World Health Organization Classification of Tumors of the Central Nervous System,” Cancer 128, no. 1 (2022): 47–58. - PubMed
Publication types
MeSH terms
Grants and funding
- 82172989/National Natural Science Foundation of China
- 82260524/National Natural Science Foundation of China
- 82273068/National Natural Science Foundation of China
- GJJ210177/Jiangxi Province Department of Education Science and Technology Research Project, China
- 20204343/Project Fund of Jiangxi Provincial Health Commission
- 202130873/Project Fund of Jiangxi Provincial Health Commission
- 202210044/Project Fund of Jiangxi Provincial Health Commission
- 20212BCJ23023/Jiangxi Training Program for Academic and Technical Leaders of Major Disciplines-Young Talents Program
- 20212BBG73021/Key Research and Development Projects in Jiangxi
- 20232BAB206101/Natural Science Foundation of Jiangxi Province
- 2023ZD003/Key Project of Science and Technology Innovation of Health Commission
LinkOut - more resources
Full Text Sources
Medical

