Advancing Thalamic Nuclei Segmentation: The Impact of Compressed Sensing on MRI Processing
- PMID: 39722224
- PMCID: PMC11669628
- DOI: 10.1002/hbm.70120
Advancing Thalamic Nuclei Segmentation: The Impact of Compressed Sensing on MRI Processing
Abstract
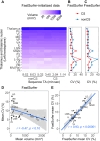
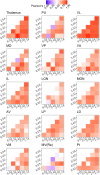
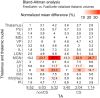
The thalamus is a collection of gray matter nuclei that play a crucial role in sensorimotor processing and modulation of cortical activity. Characterizing thalamic nuclei non-invasively with structural MRI is particularly relevant for patient populations with Parkinson's disease, epilepsy, dementia, and schizophrenia. However, severe head motion in these populations poses a significant challenge for in vivo mapping of thalamic nuclei. Recent advancements have leveraged the compressed sensing (CS) framework to accelerate structural MRI acquisition times in MPRAGE sequence variants, while fast segmentation tools like FastSurfer have reduced processing times in neuroimaging research. In this study, we evaluated thalamic nuclei segmentations derived from six different MPRAGE variants with varying degrees of CS acceleration (from about 9 to about 1-min acquisitions). Thalamic segmentations were initialized from either FastSurfer or FreeSurfer, and the robustness of the thalamic nuclei segmentation tool to different initialization inputs was evaluated. Our findings show minimal sequence effects with no systematic bias, and low volume variability across sequences for the whole thalamus and major thalamic nuclei. Notably, CS-accelerated sequences produced less variable volumes compared to non-CS sequences. Additionally, segmentations of thalamic nuclei initialized from FastSurfer and FreeSurfer were highly comparable. We provide the first evidence supporting that a good segmentation quality of thalamic nuclei with CS T1-weighted image acceleration in a clinical 3T MRI system is possible. Our findings encourage future applications of fast T1-weighted MRI to study deep gray matter. CS-accelerated sequences and rapid segmentation methods are promising tools for future studies aiming to characterize thalamic nuclei in vivo at 3T in both healthy individuals and clinical populations.
Keywords: FastSurfer; FreeSurfer; compressed sensing MRI; fast MRI acquisition; segmentation; thalamic nuclei; volumetric characterization.
© 2024 The Author(s). Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
Tobias Kober and Tom Hilbert are employed by Siemens Healthineers International AG, Switzerland.
Figures


References
-
- Alonso, J. , Pareto D., Alberich M., et al. 2021. “Quantitative Comparison of Subcortical and Ventricular Volumetry Derived From MPRAGE and MP2RAGE Images Using Different Brain Morphometry Software.” Magnetic Resonance Materials in Physics, Biology and Medicine 34, no. 6: 903–914. - PubMed
-
- Arend, I. , Henik A., and Okon‐Singer H.. 2015. “Dissociating Emotion and Attention Functions in the Pulvinar Nucleus of the Thalamus.” Neuropsychology 29, no. 2: 191–196. - PubMed
-
- Behrens, T. E. , Johansen‐Berg H., Woolrich M. W., et al. 2003. “Non‐Invasive Mapping of Connections Between Human Thalamus and Cortex Using Diffusion Imaging.” Nature Neuroscience 6, no. 7: 750–757. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

