Network Pharmacology and Metabolomics Reveal Anti-Ferroptotic Effects of Curcumin in Acute Kidney Injury
- PMID: 39722679
- PMCID: PMC11669278
- DOI: 10.2147/DDDT.S486286
Network Pharmacology and Metabolomics Reveal Anti-Ferroptotic Effects of Curcumin in Acute Kidney Injury
Abstract
Introduction: Acute kidney injury (AKI) is linked to high rates of mortality and morbidity worldwide thereby posing a major public health problem. Evidences suggest that ferroptosis is the primary cause of AKI, while inhibition of monoamine oxidase A(MAOA) and 5-hydroxytryptamine were recognized as the defender of ferroptosis. Curcumin (Cur) is a natural polyphenol and the main bioactive compound of Curcuma longa, which has been found nephroprotection in AKI. However, the potential mechanism of Cur in alleviating AKI ferroptosis remains unknown.
Objective: This study aims to investigate the effects of Cur on AKI ferroptosis.
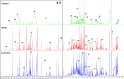
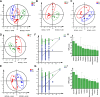
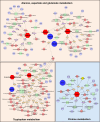
Methods: Folic acid (FA)-induced AKI mouse model and erastin/(rsl-3)-induced HK-2 model were constructed to assess the renoprotection of Cur. The nuclear magnetic resonance (NMR)-based metabolomics coupled network pharmacology approach was used to explore the metabolic regulation and potential targets of Cur. Molecular docking and enzyme activity assay was carried out to evaluate the effects of Cur on MAOA.
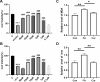
Results: Our results showed that in vivo Cur preserved renal functions in AKI mice by lowering levels of serum creatinine, blood urea nitrogen, while in vitro ameliorated the cell viability of HK-2 cells damaged by ferroptosis. Mechanistic studies indicated that Cur protected AKI against ferroptosis via inhibition of MAOA thereby regulating 5-hydroxy-L-tryptophan metabolism.
Conclusion: Our study for the first time clarified that Cur might acts as a MAOA inhibitor and alleviates ferroptosis in AKI mice, laying a scientific foundation for new insights of clinical therapy on AKI.
Keywords: acute kidney injury; curcumin; ferroptosis; metabolomics; monoamine oxidase A; network pharmacology.
© 2024 Liu et al.
Conflict of interest statement
The authors reported no conflicts of interest in this work.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

