Regulated Cell Death of Alveolar Macrophages in Acute Lung Inflammation: Current Knowledge and Perspectives
- PMID: 39722732
- PMCID: PMC11669335
- DOI: 10.2147/JIR.S497775
Regulated Cell Death of Alveolar Macrophages in Acute Lung Inflammation: Current Knowledge and Perspectives
Abstract
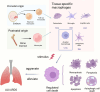
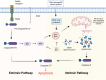
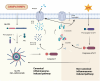
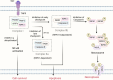
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) is a common and serious clinical lung disease characterized by extensive alveolar damage and inflammation leading to impaired gas exchange. Alveolar macrophages (AMs) maintain homeostatic properties and immune defenses in lung tissues. Several studies have reported that AMs are involved in and regulate ALI/ARDS onset and progression via different regulated cell death (RCD) programs, such as pyroptosis, apoptosis, autophagic cell death, and necroptosis. Notably, the effects of RCD in AMs in disease are complex and variable depending on the environment and stimuli. In this review, we provide a comprehensive perspective on how regulated AMs death impacts on ALI/ARDS and assess its potential in new therapeutic development. Additionally, we describe the crosstalk between different RCD types in ALI, and provide new perspectives for the treatment of ALI/ARDS and other severe lung diseases.
Keywords: acute lung injury; alveolar macrophage; apoptosis; autophagic cell death; necroptosis; pyroptosis.
© 2024 Xia et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

