The CSF p-tau/β-amyloid 42 ratio correlates with brain structure and fibrillary β-amyloid deposition in cognitively unimpaired individuals at the earliest stages of pre-clinical Alzheimer's disease
- PMID: 39723106
- PMCID: PMC11668178
- DOI: 10.1093/braincomms/fcae451
The CSF p-tau/β-amyloid 42 ratio correlates with brain structure and fibrillary β-amyloid deposition in cognitively unimpaired individuals at the earliest stages of pre-clinical Alzheimer's disease
Abstract
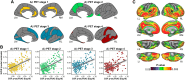
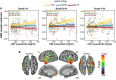
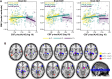
CSF concentrations of β-amyloid 42 (Aβ42) and phosphorylated tau (p-tau) are well-established biomarkers of Alzheimer's disease and have been studied in relation to several neuropathological features both in patients and in cognitively unimpaired individuals. The CSF p-tau/Aβ42 ratio, a biomarker combining information from both pathophysiological processes, has emerged as a promising tool for monitoring disease progression, even at pre-clinical stages. Here, we studied the association between the CSF p-tau/Aβ42 ratio with downstream markers of pre-clinical Alzheimer's disease progression including brain structure, glucose metabolism, fibrillary Aβ deposition and cognitive performance in 234 cognitively unimpaired individuals, who underwent cognitive testing, a lumbar puncture, MRI, 18F-fluorodeoxyglucose and 18F-flutemetamol PET scanning. We evaluated both main effects and interactions with Alzheimer's disease risk factors, such as older age, female sex and the apoliporoptein E (APOE)-ɛ4 allele, in a priori defined regions of interest and further examined the associations on the whole-brain using voxel-wise regressions. In addition, as the association between CSF Alzheimer's disease biomarkers and brain structure and function may be non-linear, we tested the interaction between the CSF p-tau/Aβ42 ratio and stages of pre-clinical Alzheimer's disease defined using the amyloid (A) and tau (T) classification. We found significantly positive associations between CSF p-tau/Aβ42 and both cortical Aβ deposition and regional grey matter volume while no effect was observed for brain metabolism. A significant interaction with age indicated that, for the same level of CSF p-tau/Aβ42, older individuals displayed both increased Aβ deposition and lower grey matter volume, in widespread cortical areas. In addition, we found that women compared with men had a greater Aβ fibrillary accumulation in midline cortical areas and inferior temporal regions, for the same level of the CSF biomarker. The impact of CSF p-tau/Aβ42 on grey matter volume was modulated by AT stages, with A+T+ individuals displaying significantly less positive associations in areas of early atrophy in the Alzheimer's continuum. Finally, we found that sex and APOE-ɛ4 modulated the association between the CSF biomarker and episodic memory as well as abstract reasoning, respectively. Our data indicate that the CSF p-tau/Aβ42 ratio is strongly associated with multiple downstream neuropathological events in cognitively unimpaired individuals and may thus serve as a potent biomarker to investigate the earliest changes in pre-clinical Alzheimer's disease. Given that its impact on both Aβ deposition and grey matter volume is modulated by specific risk factors, our results highlight the need to take into account such predisposing variables in both clinical practice and prevention trials.
Keywords: Alzheimer’s disease; CSF biomarkers; amyloid deposition; brain structure; p-tau/Aβ42 ratio.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant, at advisory boards or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. M.S.-C. has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and has given lectures in symposia sponsored by Roche Diagnostics, S.L.U and Roche Farma, S.A. M.S.-C. was granted with the project ‘Sex and gender role in pre-clinical Alzheimer’s disease: from pathophysiology to clinical trials inclusion’, funded by Roche Diagnostics International Ltd; payments were made to the institution (BBRC).
Figures



References
-
- Chételat G, Villemagne VL, Pike KE, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133(11):3349–3358. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
