Biocompatible Iron Oxide Nanoparticles Display Antiviral Activity Against Two Different Respiratory Viruses in Mice
- PMID: 39723174
- PMCID: PMC11669338
- DOI: 10.2147/IJN.S475323
Biocompatible Iron Oxide Nanoparticles Display Antiviral Activity Against Two Different Respiratory Viruses in Mice
Abstract
Background: Severe Acute Respiratory syndrome coronavirus 2 (SARS-CoV-2) and Influenza A viruses (IAVs) are among the most important causes of viral respiratory tract infections, causing similar symptoms. IAV and SARS-CoV-2 infections can provoke mild symptoms like fever, cough, sore throat, loss of taste or smell, or they may cause more severe consequences leading to pneumonia, acute respiratory distress syndrome or even death. While treatments for IAV and SARS-CoV-2 infection are available, IAV antivirals often target viral proteins facilitating the emergence of drug-resistant viral variants. Hence, universal treatments against coronaviruses and IAVs are hard to obtain due to genus differences (in the case of coronavirus) or subtypes (in the case of IAV), highlighting the need for novel antiviral therapies. Interestingly, iron oxide nanoparticles (IONPs) with a 10 nm core size and coated with the biocompatible dimercaptosuccinic acid (DMSA: DMSA-IONP-10) display antiviral activity against SARS-CoV-2 in vitro.
Methods: We analyzed the antiviral activity of DMSA-IONP-10 against SARS-CoV-2 infection in vivo, and against IAV infection in vitro and in vivo.
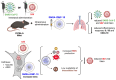
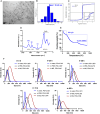
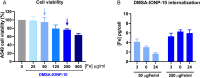
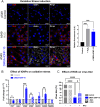
Results: DMSA-IONP-10 treatment of mice after SARS-CoV-2 infection impaired virus replication in the lungs and led to a mildly reduced pro-inflammatory cytokine induction after infection, indicating that these IONPs can serve as COVID-19 therapeutic agents. These IONPs also had a prophylactic and therapeutic effect against IAV in tissue cultured cells at non-cytotoxic doses, and a therapeutic effect in IAV-infected-mice, inhibiting viral replication and slightly dampening the inflammatory response after viral infection. As an exacerbated inflammatory response to IAVs and SARS-CoV-2 is detrimental to the host, weakening this response in mice through IONP treatment may reduce disease severity. Interestingly, our data suggest that IONP treatment affects oxidative stress and iron metabolism in cells, which may influence IAV production.
Conclusion: This study highlights the antiviral activity of DMSA-IONP-10 against important human respiratory viruses.
Keywords: IAV; SARS-CoV-2; iron metabolism; iron oxide nanoparticles; oxidative stress; viral infection.
© 2024 DeDiego et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- COVID-19 epidemiological update – 6 November 2024. Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update-.... Accessed November 11, 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

