Phage-resistance alters Lipid A reactogenicity: a new strategy for LPS-based conjugate vaccines against Salmonella Rissen
- PMID: 39723217
- PMCID: PMC11668645
- DOI: 10.3389/fimmu.2024.1450600
Phage-resistance alters Lipid A reactogenicity: a new strategy for LPS-based conjugate vaccines against Salmonella Rissen
Abstract
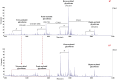
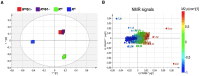
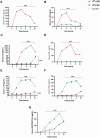
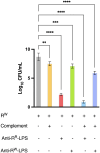
Salmonella enterica serovar Rissen (S. Rissen) is an emerging causative agent of foodborne diseases. The current emergence of antibiotic resistance makes necessary alternative therapeutic strategies. In this study, we investigated the potential of a phage-resistant strain of S. Rissen (RR) as a tool for developing an effective lipopolysaccharide (LPS)-based vaccine. The LPS O-antigen is known to play critical roles in protective immunity against Salmonella. However, the high toxicity of the LPS lipid A moiety limits its use in vaccines. Here, we demonstrated that the acquisition of bacteriophage resistance by S. Rissen leads to structural modifications in the LPS structure. Using NMR and mass spectrometry, we characterized the LPS from phage-resistant strains as a smooth variant bearing under-acylated Lipid A portions (penta- and tetra-acylated forms). We then combined RT-qPCR and NMR-based metabolomics to explore the effects of phage resistance and LPS modification on bacterial fitness and virulence. Finally, we conducted in vivo studies to determine whether lysogeny-induced remodeling of LPS affects the host immune response. Results revealed that the under-acylated variant of LPS from RR attenuates the inflammatory response in BALB/c mice, while eliciting a specific antibody response that protects against S. Rissen (RW) infection. In conclusion, our findings suggest that phage resistance, through lipid A modification, may offer a novel strategy for reducing LPS toxicity, highlighting its potential as a promising biological approach for developing LPS-based vaccines against Salmonella infections.
Keywords: bacteriophages; host immune response; lipid A; salmonella infection; vaccine.
Copyright © 2024 Cuomo, Medaglia, Casillo, Gentile, Fruggiero, Corsaro and Capparelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

