Frataxin is essential for zebrafish embryogenesis and pronephros formation
- PMID: 39723241
- PMCID: PMC11669007
- DOI: 10.3389/fcell.2024.1496244
Frataxin is essential for zebrafish embryogenesis and pronephros formation
Abstract
Background and objectives: Friedreich's Ataxia (FRDA) is a genetic disease that affects a variety of different tissues. The disease is caused by a mutation in the frataxin gene (FXN) which is important for the synthesis of iron-sulfur clusters. The primary pathologies of FRDA are loss of motor control and cardiomyopathy. These occur due to the accumulation of reactive oxygen species (ROS) in the brain and the heart due to their high metabolic rates. Our research aims to understand how developmental processes and the kidney are impacted by a deficiency of FXN.
Methods: We utilized an antisense oligomer, or morpholino, to knockdown the frataxin gene (fxn) in zebrafish embryos. Knockdown was confirmed via RT-PCR, gel electrophoresis, and Sanger sequencing. To investigate phenotypes, we utilized several staining techniques including whole mount in situ hybridization, Alcian blue, and acridine orange, as well as dextran-FITC clearance assays.
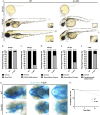
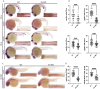
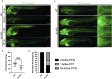
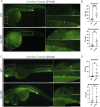
Results: fxn deficient animals displayed otolith malformations, edema, and reduced survival. Alcian blue staining revealed craniofacial defects in fxn deficient animals, and gene expression studies showed that the pronephros, or embryonic kidney, had several morphological defects. We investigated the function of the pronephros through clearance assays and found that the renal function is disrupted in fxn deficient animals in addition to proximal tubule endocytosis. Utilizing acridine orange staining, we found that cell death is a partial contributor to these phenotypes.
Discussion and conclusion: This work provides new insights about how fxn deficiency impacts development and kidney morphogenesis. Additionally, this work establishes an additional model system to study FRDA.
Keywords: development; frataxin; kidney; metabolism; nephron; zebrafish.
Copyright © 2024 Ercanbrack, Dungan, Gaul, Ramirez, J. DelVecchio, Grass and Wingert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Anheim M., Fleury M., Monga B., Laugel V., Chaigne D., Rodier G., et al. (2010). Epidemiological, clinical, paraclinical and molecular study of a cohort of 102 patients affected with autosomal recessive progressive cerebellar ataxia from Alsace, Eastern France: implications for clinical management. Neurogenetics 11 (1), 1–12. 10.1007/s10048-009-0196-y - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

