Complex and Dynamic Gene-by-Age and Gene-by-Environment Interactions Underlie Functional Morphological Variation in Adaptive Divergence in Arctic Charr (Salvelinus alpinus)
- PMID: 39723482
- PMCID: PMC11670044
- DOI: 10.1111/ede.70000
Complex and Dynamic Gene-by-Age and Gene-by-Environment Interactions Underlie Functional Morphological Variation in Adaptive Divergence in Arctic Charr (Salvelinus alpinus)
Abstract
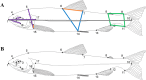
The evolution of adaptive phenotypic divergence requires heritable genetic variation. However, it is underappreciated that trait heritability is molded by developmental processes interacting with the environment. We hypothesized that the genetic architecture of divergent functional traits was dependent on age and foraging environment. Thus, we induced plasticity in full-sib families of Arctic charr (Salvelinus alpinus) morphs from two Icelandic lakes by mimicking prey variation in the wild. We characterized variation in body shape and size at two ages and investigated their genetic architecture with quantitative trait locus (QTL) analysis. Age had a greater effect on body shape than diet in most families, suggesting that development strongly influences phenotypic variation available for selection. Consistent with our hypothesis, multiple QTL were detected for all traits and their location depended on age and diet. Many of the genome-wide QTL were located within a subset of duplicated chromosomal regions suggesting that ancestral whole genome duplication events have played a role in the genetic control of functional morphological variation in the species. Moreover, the detection of two body shape QTL after controlling for the effects of age provides additional evidence for genetic variation in the plastic response of morphological traits to environmental variation. Thus, functional morphological traits involved in phenotypic divergence are molded by complex genetic interactions with development and environment.
Keywords: development; genetic architecture; phenotypic divergence; phenotypic plasticity; salmonid fishes.
© 2024 The Author(s). Evolution & Development published by Wiley Periodicals LLC.
Figures



References
-
- Adams, D. C. , Collyer M. L., Kaliontzopoulou A., and Baken E. K.. 2021. Geomorph: Software for Geometric Morphometric Analyses. R package version 4.0. https://cran.r-project.org/package=geomorph.
-
- Allendorf, F. W. , and Thorgaard G. H.. 1984. “Tetraploidy and the Evolution of Salmonid Fishes.” In Evolutionary Genetics of Fishes, edited by Turner B. J., 1–53. Boston, MA: Springer.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

